Chemistry:Sulfur dibromide
From HandWiki
| |
| Names | |
|---|---|
IUPAC name
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
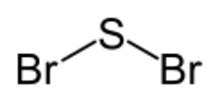
| SBr 2 | |
| Molar mass | 191.873 g/mol |
| Appearance | gas |
| Structure | |
| C2v | |
| Bent | |
| Hazards | |
| Safety data sheet | ICSC 1661 |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| Related compounds | |
Related
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfur dibromide is the chemical compound with the formula SBr
2. It is a toxic gas.
Sulfur dibromide readily decomposes into S
2Br
2 and elemental bromine. In analogy to sulfur dichloride, it hydrolyzes in water to give hydrogen bromide, sulfur dioxide and elemental sulfur.
SBr
2 can be prepared by reacting SCl
2 with HBr, but due to its rapid decomposition it cannot be isolated at standard conditions. Instead, the more stable S
2Br
2 is obtained.[1]
References
- ↑ Wiberg, Egon; Holleman, A. F.; Wiberg, Nils (2001). Inorganic Chemistry. Academic Press. p. 528. ISBN 978-0-12-352651-9. https://books.google.com/books?id=Mtth5g59dEIC&pg=PA529.
 |

