Chemistry:Thionyl fluoride
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Thionyl fluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| F2OS | |||
| Molar mass | 86.06 g·mol−1 | ||
| Appearance | colorless gas | ||
| Melting point | −110.5 °C (−166.9 °F; 162.7 K) | ||
| Boiling point | −43.8 °C (−46.8 °F; 229.3 K) | ||
| hydrolysis | |||
| Solubility | soluble in ethanol, ether, benzene | ||
| Vapor pressure | 75.7 kPa (-50 °C)[1] | ||
| Structure | |||
| trigonal pyramidal | |||
| Thermochemistry | |||
Std molar
entropy (S |
278.6 J/mol·K[2] | ||
Std enthalpy of
formation (ΔfH⦵298) |
-715 kJ/mol[2] | ||
Std enthalpy of
combustion (ΔcH⦵298) |
56.8 J/mol·K[2] | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H300, H310, H314, H330 | |||
| P260, P262, P264, P270, P271, P280, P284, P301+310, P301+330+331, P302+350, P303+361+353, P304+340, P305+351+338, P310, P320, P321, P322, P330, P361, P363, P403+233, P405, P501 | |||
| Related compounds | |||
Related oxohalides
|
Thionyl chloride Thionyl bromide | ||
Related compounds
|
Nitrosyl fluoride Carbonyl fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
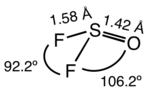
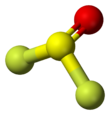
Thionyl fluoride is the inorganic compound with the formula SOF2. This colourless gas is mainly of theoretical interest, but it is a product of the degradation of sulfur hexafluoride, an insulator in electrical equipment. The molecule adopts a distorted pyramidal structure, with Cs symmetry. The S-O and S-F distances are 1.42 and 1.58 Å, respectively. The O-S-F and F-S-F angles are 106.2 and 92.2°, respectively. Thionyl chloride and thionyl bromide have similar structures, although these compounds are liquid at room temperature. Mixed halides are also known, such as SOClF, thionyl chloride fluoride.[3]
Synthesis and reactions
Thionyl fluoride can be produced by the reaction of thionyl chloride with fluoride sources such as antimony trifluoride.[4][5]
Alternatively, it arises via the fluorination of sulfur dioxide:[5]
- SO2 + PF5 → SOF2 + POF3
Thionyl fluoride arises as a fleeting intermediate from the decomposition of sulfur hexafluoride as the result of electrical discharges which generate sulfur tetrafluoride. SF4 hydrolyzes to give thionyl fluoride, which in turn hydrolyzes further as described below.[6]
As expected from the behavior of the other thionyl halides, this compound hydrolyzes readily, giving hydrogen fluoride and sulfur dioxide:[5]
- SOF2 + H2O → 2 HF + SO2
In contrast to thionyl chloride and bromide, thionyl fluoride is not useful for halogenation. The related derivative, sulfur tetrafluoride is however useful for that purpose.
References
- ↑ Thionyl fluoride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-05-11)
- ↑ 2.0 2.1 2.2 "Thionyl fluoride". http://chemister.ru/Database/properties-en.php?dbid=1&id=8280.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
- ↑ W. C. Smith, E. L. Muetterties "Thionyl Fluoride" Inorganic Syntheses 1960, Volume 6, pages: 162-163. doi:10.1002/9780470132371.ch50
- ↑ 5.0 5.1 5.2 Holleman, Arnold F. (2001). Inorganic Chemistry. Academic Press. pp. 542. ISBN 978-0-12-352651-9. https://books.google.com/books?id=LxhQPdMRfVIC&q=%22Thionyl+fluoride%22&pg=PA542. Retrieved 2008-07-29.
- ↑ Pepi, Federico; Andreina Ricci; Marco Di Stefano; Marzio Rosi; Giuseppe D'Arcangelo (September 18, 2002). "Thionyl Fluoride from Sulfur Hexafluoride Corona Discharge Decomposition: Gas-Phase Chemistry of (SOF2)H+ Ions". Journal of Physical Chemistry A 106 (40): 9261–9266. doi:10.1021/jp021074v. Bibcode: 2002JPCA..106.9261P. http://pubs.acs.org/cgi-bin/abstract.cgi/jpcafh/2002/106/i40/abs/jp021074v.html.
External links
 |