Chemistry:Thallium(III) acetate
| |
| Names | |
|---|---|
| IUPAC name
Thallium(III) acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
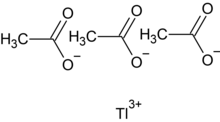
| Tl(C2H3O2)3 | |
| Molar mass | 381.52 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thallium(III) acetate is the acetate salt of thallium, with the chemical formula Tl(CH3COO)3. As a selective culture medium in microbiology,[1] thallium acetate is toxic,[2] but it can also be used as a hair loss agent. A dose of 8 mg/kg will cause acute poisoning, and the minimum lethal dose for adults is 12 mg/kg.[3]
Preparation
Thallium acetate can be obtained by reacting 80% acetic acid with thallium(III) oxide, and the product crystallizes in acetic anhydride.[4]
Properties
Anhydrous thallium(III) acetate crystallises in the monoclinic system with space group C2/c. The unit cell dimensions are a = 15.54 Å b = 8.630 Å and c = 7.848 Å with β = 113.92°. There are four formula per unit cell. and density is 2.57. Three acetate ions are chellated to each thallium ion.[5]
Thallium(III) acetate monohydrate also crystallises in the monoclinic system with space group C2/c, a = 9.311 Å, b = 14.341 Å, c = 9.198 Å, β = 119.69 °. Unit cell volume is V = 1067.0 Å3 Z = 4, density is 2.49.[6]
References
- ↑ Bulich, Anthony A.; Hartman, Paul A. (Nov 1969). "Evaluation of Thallium Acetate-Citrate Medium for Isolation of Enterococci". Applied Microbiology 18 (5): 944–945. doi:10.1128/am.18.5.944-945.1969. ISSN 0003-6919. PMID 5370465. PMC 378124. http://dx.doi.org/10.1128/am.18.5.944-945.1969.
- ↑ World Health Organization (2008). Anthrax in humans and animals. World Health Organization. pp. 139–. ISBN 978-92-4-154753-6. https://books.google.com/books?id=EKYihvnaA7oC&pg=PA139. Retrieved 23 February 2011.
- ↑ "铊、玻璃和人——从两桩铊中毒事件说起". 中国科学院上海硅酸盐研究所. http://119.78.242.243:8080/kexie/news.asp?id=144. Retrieved 2015-05-20.
- ↑ Kolling, Orland W.; Mawdsley, Elizabeth A. (1971). "Anhydrous Thallium(III) Acetate". Transactions of the Kansas Academy of Science 74 (1): 38. doi:10.2307/3627666. https://www.jstor.org/stable/3627666.
- ↑ Faggiani, R.; Brown, I. D. (1 September 1978). "Thallium(III) triacetate". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 34 (9): 2845–2846. doi:10.1107/S0567740878009358. Bibcode: 1978AcCrB..34.2845F.
- ↑ Faggiani, R.; Brown, I. D. (15 September 1982). "Thallium triacetate monohydrate". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 38 (9): 2473–2475. doi:10.1107/S0567740882009091. Bibcode: 1982AcCrB..38.2473F.
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |

