Chemistry:Thallium(I) fluoride
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Thallium(I) fluoride | |
| Other names
Thallium monofluoride
Thallous fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| TlF | |
| Molar mass | 223.3817 g/mol |
| Appearance | White crystals |
| Density | 8.36 g cm−3 |
| Melting point | 327 °C (621 °F; 600 K) |
| Boiling point | 655 °C (1,211 °F; 928 K) (decomposes) |
| 78.6 g/100 mL (at 15 °C)[1] | |
| Solubility | slightly soluble in ethanol |
| −44.4·10−6 cm3/mol | |
| Structure | |
| Orthorhombic, oP8 | |
| Fmmm, No. 28 | |
| Hazards[2] | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+310, P304+340, P310, P314, P320, P321, P330, P391, P403+233, P405, P501 | |
| Related compounds | |
Other anions
|
Thallium(I) chloride |
Other cations
|
Gallium(III) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
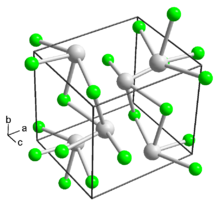
Thallium(I) fluoride is the inorganic compound with the formula TlF. It is a white solid, forming orthorhombic crystals. The solid is slightly deliquescent.[1] It has a distorted sodium chloride (rock salt) crystal structure,[3][4] due to the 6s2 inert pair on Tl+.[5]
This salt is unusual among the thallium(I) halides in that it is very soluble in water.[6]
Reactions
Thallium(I) fluoride can be prepared by the reaction of thallium(I) carbonate with hydrofluoric acid.[3]
References
- ↑ 1.0 1.1 Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, pp. 407, ISBN 0-8493-8671-3, https://books.google.com/books?id=0fT4wfhF1AsC&pg=PA407, retrieved 2008-06-17
- ↑ "399833 Thallium(I) fluoride 99%". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/399833.
- ↑ 3.0 3.1 Wiberg, Nils; Wiberg, Egon; Holleman, A. F. (2001), Inorganic Chemistry, Academic Press, pp. 1037, ISBN 0-12-352651-5, https://books.google.com/books?id=LxhQPdMRfVIC&q=%22Thallium(I)+fluoride%22&pg=PA1037, retrieved 2008-06-17
- ↑ Meyer, Gerd; Naumann, Dieter; Wesemann, Lars (2006), Inorganic Chemistry in Focus III, Wiley-VCH, pp. 21, ISBN 3-527-31510-1, https://books.google.com/books?id=5y41VGaZEFIC&q=%22Thallium(I)+fluoride%22&pg=PA21, retrieved 2008-06-17
- ↑ Berastegui, P.; Hull, S. (2000). "The Crystal Structures of Thallium(I) Fluoride". Journal of Solid State Chemistry 150 (2): 266. doi:10.1006/jssc.1999.8587. Bibcode: 2000JSSCh.150..266B.
- ↑ Arora, M. G. (2003), P-block Elements, Anmol Publications, pp. 35, ISBN 81-7488-563-3, https://books.google.com/books?id=QR3TCaKaykEC&q=%22Thallium(I)+fluoride%22&pg=PA35, retrieved 2008-06-17
 |
