Chemistry:Strontium acetate
From HandWiki
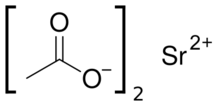
| |
| Names | |
|---|---|
| IUPAC name
Strontium acetate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| Sr(C2H4O2)2 | |
| Molar mass | 205.932 g/mol |
| Appearance | White crystals |
| Density | 2.099 g/cm3 |
| Melting point | 150 °C (302 °F; 423 K) |
| Soluble | |
| log P | −1.122 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Not flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Strontium acetate is a compound of strontium. It is a white solid and is soluble in water like other acetates. It is used as a pathway for other chemicals such as barium acetate. Additionally, it is used in some strontium-containing toothpastes.[4]
Preparation
Strontium acetate is formed by reacting strontium hydroxide or strontium carbonate in acetic acid.
References
- ↑ "STRONTIUM ACETATE | 543-94-2". https://www.chemicalbook.com/ChemicalProductProperty_EN_CB7336274.htm.
- ↑ "Strontium Acetate". https://www.americanelements.com/strontium-acetate-543-94-2.
- ↑ "MFCD00036392 | C4H6O4Sr". http://www.chemspider.com/Chemical-Structure.10522.html.
- ↑ "Pictures, stories, and facts about the element Strontium in the Periodic Table". https://periodictable.com/Elements/038/index.html.
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |

