Chemistry:Thallium(III) oxide
| |
| Names | |
|---|---|
| Other names
thallium trioxide, thallium sesquioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties[1] | |
| Tl2O3 | |
| Molar mass | 456.76 g/mol |
| Appearance | dark brown solid |
| Density | 10.19 g/cm3, solid (22 °C) |
| Melting point | 717 °C (1,323 °F; 990 K) |
| Boiling point | 875 °C (1,607 °F; 1,148 K) (decomposes) |
| insoluble | |
| +76.0·10−6 cm3/mol | |
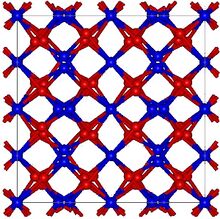
| Structure | |
| Cubic, (Bixbyite) cI80[2] | |
| Ia3 (No. 206) | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | danger |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
44 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thallium(III) oxide, also known as thallic oxide, is a chemical compound of thallium and oxygen. It occurs in nature as the rare mineral avicennite.[4] Its structure is related to that of Mn2O3 which has a bixbyite like structure. Tl2O3 is metallic with high conductivity and is a degenerate n-type semiconductor which may have potential use in solar cells.[5] A method of producing Tl2O3 by MOCVD is known.[6] Any practical use of thallium(III) oxide will always have to take account of thallium's poisonous nature. Contact with moisture and acids may form poisonous thallium compounds.
Production
It is produced by the reaction of thallium with oxygen or hydrogen peroxide in an alkaline thallium(I) solution. Alternatively, it can be created by the oxidation of thallium(I) nitrate by chlorine in an aqueous potassium hydroxide solution.[7]
References
- ↑ Weast, Robert C., ed (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B156. ISBN 0-8493-0462-8..
- ↑ Otto H.H.; Baltrasch R.; Brandt H.J. (1993). "Further evidence for Tl3+ in Tl-based superconductors from improved bond strength parameters involving new structural data of cubic Tl2O3". Physica C 215 (1–2): 205. doi:10.1016/0921-4534(93)90382-Z.
- ↑ GHS: Sigma-Aldrich 204617
- ↑ http://www.handbookofmineralogy.org/pdfs/avicennite.pdf Handbook of Mineralogy
- ↑ Phillips R. J.; Shane M. J.; Switzer J. A. (1989). "Electrochemical and photoelectrochemical deposition of Thallium(III) Oxide thin films". Journal of Materials Research 4 (4): 923. doi:10.1557/JMR.1989.0923.
- ↑ D. Berry; R. T. Holm; R. L. Mowery; N. H. Turner; M. Fatemi (1991). "Thallium(III) Oxide by Organometallic Chemical Vapor Deposition". Chemistry of Materials 3 (1): 72–77. doi:10.1021/cm00013a019.
- ↑ Georg Brauer; Handbuch der präparativen anorganischen Chemie, Band 2, S.884; ISBN:3-432-87813-3 (in German)
 |


