Chemistry:Tolrestat
From HandWiki
Short description: Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
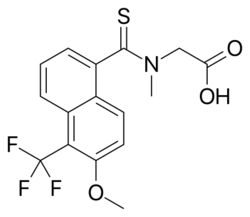
| Formula | C16H14F3NO3S |
| Molar mass | 357.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tolrestat (INN) (AY-27773) is an aldose reductase inhibitor[1] which was approved for the control of certain diabetic complications.[2]
While it was approved for marketed in several countries, it failed a Phase III trial in the U.S. due to toxicity and never received FDA approval. It was discontinued by Wyeth in 1997 because of the risk of severe liver toxicity and death. It was sold under the tradename Alredase.
References
- ↑ "N-[5-(trifluoromethyl)-6-methoxy-1-naphthalenyl]thioxomethyl]- N-methylglycine (Tolrestat), a potent, orally active aldose reductase inhibitor". J. Med. Chem. 27 (3): 255–6. March 1984. doi:10.1021/jm00369a003. PMID 6422042.
- ↑ "Aldose reductase inhibitors: a potential new class of agents for the pharmacological control of certain diabetic complications". J. Med. Chem. 28 (7): 841–9. July 1985. doi:10.1021/jm00145a001. PMID 3925146.
 |

