Chemistry:Sotagliflozin
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌsoʊtəɡlɪˈfloʊzɪn/ SOH-tə-gli-FLOH-zin |
| Trade names | Zynquista, Inpefa |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
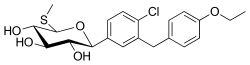
| Formula | C21H25ClO5S |
| Molar mass | 424.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sotagliflozin, sold under the brand name Inpefa among others, is a medication used to reduce the risk of death due to heart failure.[1]
The most common side effect is genital infection in women.[2] Other common side effects include diabetic ketoacidosis, diarrhea, and genital infection in men.[2]
Sotagliflozin was approved for medical use in the European Union in April 2019, as Zynquista, for the treatment for type 1 diabetes,[2] and in the United States in May 2023, to reduce the risk of death due to heart failure.[1][3] The marketing authorization for sotagliflozin was withdrawn in the EU in August 2022.[2]
Medical uses
In the United States, sotagliflozin is indicated to reduce the risk of cardiovascular death.[1] Sotaglifozin is a sodium-glucose co-transporter 1 and 2 inhibitor that reduces both postprandial glucose and insulin levels by delaying intestinal glucose absorption, decreases gastric inhibitory polypeptide, and elevations in glucagon-like peptide and peptide yy levels are consistent with local inhibition of intestinal SGLT1.[4] Combination of insulin with sotaglifozin 200 and 400 mg led to a significant lowering of systolic and diastolic blood pressure and multiple indirect markers of arterial stiffness, including pulse pressure, without changes in pulse rates.[5] Also, it decreased the incidence of myocardial infarction and stroke, pointing to a potential side effect of SGLT1 inhibition.[6]
Society and culture
Legal status
The US Food and Drug Administration (FDA) refused its approval for use in combination with insulin for the treatment of type 1 diabetes. It is developed by Lexicon Pharmaceuticals.[7][8][9]
In May 2023 the US FDA approved Inpefa (sotagliflozin), a once-daily oral tablet, to decrease the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visit in adults with heart failure or type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk.[10]
References
- ↑ 1.0 1.1 1.2 1.3 "Inpefa- sotagliflozin tablet". 5 June 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1a46614e-05f6-421a-b6f4-d6f8760d643a.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Zynquista EPAR". 27 February 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/zynquista. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Lexicon Announces FDA Approval of Inpefa (sotagliflozin) for Treatment of Heart Failure" (Press release). Lexicon Pharmaceuticals. 26 May 2023. Archived from the original on 20 October 2023. Retrieved 28 May 2023 – via GlobeNewswire.
- ↑ "Sotagliflozin Decreases Postprandial Glucose and Insulin Concentrations by Delaying Intestinal Glucose Absorption". The Journal of Clinical Endocrinology and Metabolism 105 (4): e1235–e1249. April 2020. doi:10.1210/clinem/dgz258. PMID 31837264.
- ↑ "Effect of sotagliflozin as an adjunct to insulin therapy on blood pressure and arterial stiffness in adults with type 1 diabetes: A post hoc pooled analysis of inTandem1 and inTandem2". Diabetes & Vascular Disease Research 18 (1): 1479164121995928. 2021. doi:10.1177/1479164121995928. PMID 33611925.
- ↑ "Effects of SGLT2 Inhibitors beyond Glycemic Control-Focus on Myocardial SGLT1". International Journal of Molecular Sciences 22 (18): 9852. September 2021. doi:10.3390/ijms22189852. PMID 34576016.
- ↑ "Sotagliflozin as an Adjunct to Insulin for Type 1 Diabetes". U.S. Food and Drug Administration (FDA). 17 January 2019. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM629783.pdf.
- ↑ "Sanofi: FDA advisory committee votes on Zynquista (sotagliflozin) as treatment for adults with type 1 diabetes" (Press release). Sanofi. 17 January 2019. Archived from the original on 1 August 2019. Retrieved 1 April 2019 – via GlobeNewswire.
- ↑ "Sanofi: FDA advisory committee votes on Zynquista (sotagliflozin) as treatment for adults with type 1 diabetes". Sanofi (Press release). 18 January 2019. Archived from the original on 29 November 2020. Retrieved 28 October 2020.
- ↑ https://www.uspharmacist.com/article/oncedaily-inpefa-approved-for-treating-heart-failure
Further reading
- "Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives". Cardiovascular Diabetology 18 (1): 20. February 2019. doi:10.1186/s12933-019-0828-y. PMID 30819210.
- "Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor". Diabetes & Vascular Disease Research 12 (2): 101–110. March 2015. doi:10.1177/1479164114563304. PMID 25690134.
- "Effect of Sotagliflozin on Total Hospitalizations in Patients With Type 2 Diabetes and Worsening Heart Failure : A Randomized Trial". Annals of Internal Medicine 174 (8): 1065–1072. August 2021. doi:10.7326/M21-0651. PMID 34152828. https://pure.rug.nl/ws/files/200951038/m21_0651.pdf.
External links
- Clinical trial number NCT03521934 for "Effect of Sotagliflozin on Cardiovascular Events in Participants With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF Trial)" at ClinicalTrials.gov
- Clinical trial number NCT03315143 for "Effect of Sotagliflozin on Cardiovascular and Renal Events in Participants With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED)" at ClinicalTrials.gov
 |

