Chemistry:Potassium ferrioxalate

| |
| |
| Names | |
|---|---|
| IUPAC name
Potassium iron(III) oxalate
| |
| Other names
potassium ferrioxalate
potassium trisoxalatoferrate(III) | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| |
| |
| Properties | |
| K 3[Fe(C 2O 4) 3] (anhydrous) K 3[Fe(C 2O 4) 3] · 3H2O (trihydrate) | |
| Molar mass | 437.20 g/mol (anhydrous) 491.25 g/mol (trihydrate) |
| Appearance | emerald green crystals |
| Density | 2.13 g/cm3 |
| Melting point | 230 °C (446 °F; 503 K) the trihydrate loses 3H 2O at 113 °C[1] |
| Structure | |
| octahedral | |
| 0 D | |
| Hazards[2] | |
| Main hazards | Corrosive. Eye, respiratory and skin irritant. |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312 | |
| P280, P301+330+331, P302+353Script error: No such module "Preview warning".Category:GHS errors, P312, P330, P363, P403, P501 | |
| Related compounds | |
Other anions
|
Sodium ferrioxalate |
Related compounds
|
Iron(II) oxalate Iron(III) oxalate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III)[3] is a chemical compound with the formula K
3[[[Iron|Fe]](C
2O
4)
3]. It often occurs as the trihydrate K
3[Fe(C
2O
4)
3] · 3H2O. Both are crystalline compounds, lime green in colour.[4]
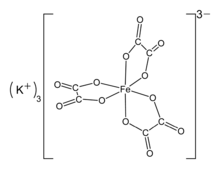
The compound is a salt consisting of ferrioxalate anions, [Fe(C
2O
4)
3]3−, and potassium cations K+. The anion is a transition metal oxalate complex consisting of an iron atom in the +3 oxidation state and three bidentate oxalate C
2O2−
4 ligands. Potassium is a counterion, balancing the −3 charge of the complex. In solution, the salt dissociates to give the ferrioxalate anion, [Fe(C
2O
4)
3]3−, which appears fluorescent green in color. The salt is available in anhydrous form[3] as well as a trihydrate.[5]
The ferrioxalate anion is quite stable in the dark, but it is decomposed by light and high-energy electromagnetic radiation.
Preparation
The complex can be synthesized by the reaction between iron(III) sulfate, barium oxalate and potassium oxalate:[4]
- Fe
2(SO
4)
3 + 3 BaC
2O
4 + 3 K
2C
2O
4 → 2 K
3[Fe(C
2O
4)
3] + 3 BaSO
4
As can be read in the reference above, iron(III) sulfate, barium oxalate and potassium oxalate are combined in water and digested for several hours on a steam bath. Oxalate ions from barium oxalate will then replace the sulfate ions in solution, removing them as BaSO
4 which can then be filtered and the pure material can be crystallized.
Structure
The structures of the trihydrate and of the anhydrous salt have been extensively studied.[5] which indicates that the Fe(III) is high spin; as the low spin complex would display Jahn–Teller distortions. The ammonium and mixed sodium-potassium salts are isomorphous, as are related complexes with Al3+, Cr3+, and V3+.
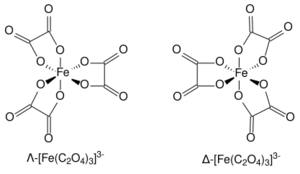
The ferrioxalate complex displays helical chirality as it can form two non-superimposable geometries. In accordance with the IUPAC convention, the isomer with the left-handed screw axis is assigned the Greek symbol Λ (lambda). Its mirror image with the right-handed screw axis is given the Greek symbol Δ (delta).[6]
Reactions
Photoreduction
The ferrioxalate anion is sensitive to light and to high-energy electromagnetic radiation, including X-rays and gamma rays. Absorption of a photon causes the decomposition of one oxalate ion to carbon dioxide CO
2 and reduction of the iron(III) atom to iron(II).[7] This photo-sensitive property is used for chemical actinometry, the measure of luminous flux, and for preparation of blueprints. This light-catalyzed redox reaction once formed the basis of some photographic processes. However due to their insensitivity and ready availability of advanced digital photography, these processes are obsolete.
Thermal decomposition
The trihydrate loses the three water molecules at 113 °C.[1]
At 296 °C, the anhydrous salt decomposes into the iron(II) complex potassium ferrioxalate, potassium oxalate, and carbon dioxide:[1]
- 2 K
3[Fe(C
2O
4)
3] → 2 K
2[Fe(C
2O
4)
2] + K
2C
2O
4 + 2 CO
2
Uses
Photometry and actinometry
The discovery of the efficient photolysis of the ferrioxalate anion was a landmark for chemical photochemistry and actinometry. The potassium salt was found to be over 1000 times more sensitive than uranyl oxalate, the compound previously used for these purposes.[7][8]
Chemistry education
The synthesis and thermal decomposition of potassium ferrioxalate is a popular exercise for high school, college or undergraduate university students, since it involves the chemistry of transition metal complexes, visually observable photochemistry, and thermogravimetry.[9]
Blueprints
Before the ready availability of wide ink-jet and laser printers, large-size engineering drawings were commonly reproduced by the cyanotype method.
That was a simple contact-based photographic process that produced a "negative" white-on-blue copy of the original drawing—a blueprint. The process is based on the photolysis of an iron(III) complex which gets converted into an insoluble iron(II) version in areas of the paper that were exposed to light.
The complex used in cyanotype is mainly ammonium iron(III) citrate, but potassium ferrioxalate is also used.[10][11]
See also
A number of other iron oxalates are known
- Iron(II) oxalate
- Iron(III) oxalate
- Sodium ferrioxalate
References
- ↑ 1.0 1.1 1.2 J. Ladriere (1992): "Mössbauer Study on the Thermal Decomposition of Potassium Tris(oxalato)ferrate(III) Trihydrate and Bis(oxalato) ferrate(II) Dihydrate". Hyperfine Interactions, volume 70, issue 1, pages 1095–1098. doi:10.1007/BF02397520
- ↑ "5936-11-8 - Potassium trioxalatoferrate(III) trihydrate - Potassium iron(III) oxalate - 31124 - Alfa Aesar". https://www.alfa.com/en/catalog/031124/.
- ↑ 3.0 3.1 A. Saritha, B. Raju, M. Ramachary, P. Raghavaiah, and K. A. Hussain (2012) "Synthesis, Crystal Structure and Characterization of Chiral, Three-Dimensional Anhydrous Potassium Tris(oxalato)ferrate(III)", Physica B: Condensed Matter, volume 407, issue 21, pages 4208-4213. doi:10.1016/j.physb.2012.07.005
- ↑ 4.0 4.1 Bailar, Jr., John C.; Jones, Eldon M. (1939). "Trioxalato Salts (Trioxalatoaluminiate, -ferriate, -chromiate, and -cobaltiate)". Inorganic Syntheses 1: 35–38. doi:10.1002/9780470132326.ch13. ISBN 9780470132326.
- ↑ 5.0 5.1 Junk, Peter C. (2005). "Supramolecular Interactions in the X-ray Crystal Structure of Potassium Tris(oxalato)ferrate(III) Trihydrate". J. Coord. Chem. 58 (4): 355–361. doi:10.1080/00958970512331334250.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 7.0 7.1 Hatchard, C. G.; Parker, C. A. (1956). "A new sensitive chemical actinometer. II. Potassium ferrioxalate as a standard chemical actinometer". Proceedings of the Royal Society of London 235 (1203): 518–36. doi:10.1098/rspa.1956.0102. Bibcode: 1956RSPSA.235..518H.
- ↑ Pozdnyakov, Ivan P.; Kel, Oksana V.; Plyusnin, Victor F.; Grivin, Vyacheslav P.; Bazhin, Nikolai M. (2008). "New Insight into Photochemistry of Ferrioxalate". J. Phys. Chem. A 112 (36): 8316–8322. doi:10.1021/jp8040583. PMID 18707071. Bibcode: 2008JPCA..112.8316P.
- ↑ John Olmsted (1984): "Preparation and analysis of potassium tris(oxalato)ferrate(III)trihydrate: A general chemistry experiment". Journal of Chemical Education, volume 61, issue 12, page 1098. doi:10.1021/ed061p1098
- ↑ Pablo Alejandro Fiorito and André Sarto Polo (2015): "A New Approach toward Cyanotype Photography Using Tris-(oxalato)ferrate(III): An Integrated Experiment". Journal of Chemical Education, volume 92, issue 10, pages 1721–1724. doi:10.1021/ed500809n
- ↑ Mike Ware (2014): Cyanomicon - History, Science and Art of Cyanotype: photographic printing in Prussian blue. Online document at www.academia.edu, published by www.mikeware.co.uk, accessed on 2019-03-29.
 |