Chemistry:Titanyl sulfate
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| O5STi | |
| Molar mass | 159.92 g·mol−1 |
| Density | 1.3984 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
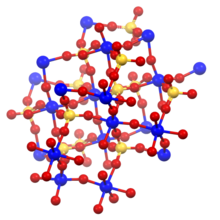
Titanyl sulfate is the inorganic compound with the formula TiOSO4. It is a white solid that forms by treatment of titanium dioxide with fuming sulfuric acid. It hydrolyzes to a gel of hydrated titanium dioxide.[1] The structure consists of dense polymeric network with tetrahedral sulfur and octahedral titanium centers. The six ligands attached to titanium are derived from four different sulfate moieties and a bridging oxide. A monohydrate is also known, being prepared similarly to the anhydrous material. In the hydrate, one Ti–OS bond is replaced by Ti–OH2.[2]
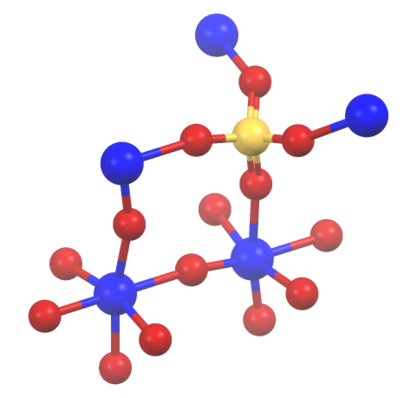
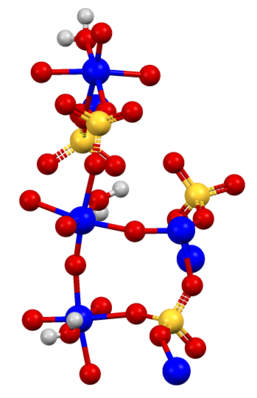
Portions of the structure of titanyl sulfate
TiOSO4, illustrating the connectivity between sulfate and titanium centers
TiOSO4(H2O), showing the presence of water
References
- ↑ Heinz Sibum; Volker Günther; Oskar Roidl; Fathi Habashi; Hans Uwe Wolf (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_095.
- ↑ Gatehouse, B. M.; Platts, S. N.; Williams, T. B. (1993). "Structure of Anhydrous Titanyl Sulfate, Titanyl Sulfate Monohydrate and Prediction of a New Structure". Acta Crystallographica Section B 49 (3): 428–435. doi:10.1107/S010876819201320X.
 |