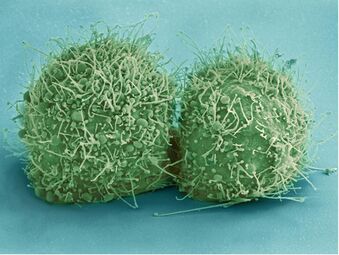
Biology:HeLa
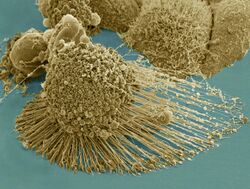

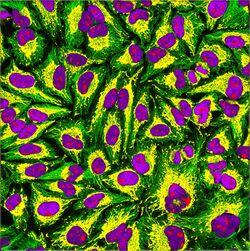
HeLa (/ˈhiːlɑː/) is an immortalized cell line used in scientific research. It is the oldest human cell line and one of the most commonly used.[1][2] HeLa cells are durable and prolific, allowing for extensive applications in scientific study.[3][4] The line is derived from cervical cancer cells taken on 8 February 1951,[5] from Henrietta Lacks, a 31-year-old African American mother of five, after whom the line is named. Lacks died of cancer on 4 October 1951.[6]
The cells from Lacks's cancerous cervical tumor were taken without her knowledge, which was common practice in the United States at the time.[7] Cell biologist George Otto Gey found that they could be kept alive,[8] and developed a cell line. Previously, cells cultured from other human cells would survive for only a few days, but cells from Lacks's tumor behaved differently.
History
Origin
In 1951, a woman named Henrietta Lacks was admitted to the Johns Hopkins Hospital with symptoms of irregular vaginal bleeding; she was subsequently treated for cervical cancer.[9] Her first treatment was performed by Lawrence Wharton Jr., who at that time collected tissue samples from her cervix without her consent.[10] Her cervical biopsy supplied samples of tissue for clinical evaluation and research by George Otto Gey, head of the Tissue Culture Laboratory. Gey's lab assistant Mary Kubicek used the roller-tube technique to culture the cells.[9] It was observed that the cells grew robustly, doubling every 20–24 hours, unlike previous specimens, which died out.[11]
The cells were propagated by Gey shortly before Lacks died of her cancer in 1951. This was the first human cell line to prove successful in vitro, which was a scientific achievement with profound future benefit to medical research. Gey freely donated these cells, along with the tools and processes that his lab developed, to any scientist requesting them, simply for the benefit of science. Neither Lacks nor her family gave permission to harvest the cells.[12] The cells were later commercialized, although never patented in their original form. There was no requirement at that time to inform patients or their relatives about such matters, because discarded material or material obtained during surgery, diagnosis, or therapy was the property of the physician or the medical institution.[citation needed]
As was customary for Gey's lab assistant, the culture was named after the first two letters of Henrietta Lacks' first and last names, He + La.[9] Before a leak to the public in the 1970s, which revealed her true name, the "HeLa" cell line was mistakenly believed to have been named after a "Helen Lane" or "Helen Larson".[4][13]
When other cell cultures were being invaded by suspected HeLa cells, one research group contacted the Lacks family,[when?] seeking DNA samples to help identify contaminating cell lines. The family never understood the purpose of the visit, but they were distressed by their understanding of what the researchers told them.[13][14] These cells are treated as cancer cells, as they are descended from a biopsy taken from a visible lesion on the cervix as part of Lacks's diagnosis of cancer.[15]
HeLa cells, like other cell lines, are termed "immortal" because they can divide an unlimited number of times in a laboratory cell culture plate, as long as fundamental cell survival conditions are met (i.e. being maintained and sustained in a suitable environment). There are many strains of HeLa cells, because they mutate during division in cell cultures, but all HeLa cells are descended from the same tumor cells removed from Lacks. The total number of HeLa cells that have been propagated in cell culture far exceeds the total number of cells that were in Henrietta Lacks's body.[16]
Controversy
Lacks's case is one of many examples of the lack of informed consent in 20th-century medicine. Communication between tissue donors and doctors was virtually nonexistent—cells were taken without patient consent, and patients were not told what the cells would be used for. Johns Hopkins Hospital, where Lacks received treatment and had her tissue harvested, was the only hospital in the Baltimore area where African American patients could receive free care. The patients who received free care from this segregated sect of the hospital often became research subjects without their knowledge.[17] Lacks's family also had no access to her patient files and had no say in who received HeLa cells or what they would be used for. Additionally, as HeLa cells were popularized and used more frequently throughout the scientific community, Lacks's relatives received no financial benefit and continued to live with limited access to healthcare.[18][13]
This issue of who owns tissue samples taken for research was brought up in the Supreme Court of California case of Moore v. Regents of the University of California in 1990. The court ruled that a person's discarded tissue and cells are not his or her property and can be commercialized.[19]
Lacks's case influenced the establishment of the Common Rule in 1981. The Common Rule enforces informed consent by ensuring that doctors inform patients if they plan to use any details of the patient's case in research and give them the choice of disclosing the details or not. Tissues connected to their donors' names are also strictly regulated under this rule, and samples are no longer named using donors' initials, but rather by code numbers.[19] To further resolve the issue of patient privacy, Johns Hopkins established a joint committee with the NIH and several of Lacks's family members to determine who receives access to Henrietta Lacks's genome.[20]
In 2021, Henrietta Lacks's estate sued to get past and future payments for the alleged unauthorized and widely known sale of HeLa cells by Thermo Fisher Scientific.[21] Lacks's family hired an attorney to seek compensation from upwards of 100 pharmaceutical companies that have used and profited from HeLa cells.[22] Settlement of the suit with Thermo Fisher Scientific was announced in August 2023, with undisclosed terms.[23] Subsequent to the settlement it was announced that the Lacks family was suing the company Ultragenyx.[24]
Use in research
HeLa cells were the first human cells to be successfully cloned in 1953, by Theodore Puck and Philip I. Marcus at the University of Colorado, Denver.[25] Since then, HeLa cells have "continually been used for research into cancer, AIDS, the effects of radiation and toxic substances, gene mapping, and countless other scientific pursuits."[26] According to author Rebecca Skloot, by 2009, "more than 60,000 scientific articles had been published about research done on HeLa [cells], and that number was increasing steadily at a rate of more than 300 papers each month."[19]
Polio eradication
HeLa cells were used by Jonas Salk to test the first polio vaccine in the 1950s. They were observed to be easily infected by the poliomyelitis virus, causing infected cells to die.[5] This made HeLa cells highly desirable for polio vaccine testing, since results could be easily obtained. A large volume of HeLa cells were needed for the testing of Salk's polio vaccine, prompting the National Foundation for Infantile Paralysis (NFIP) to find a facility capable of mass-producing HeLa cells.[27] In the spring of 1953, a cell culture factory was established at Tuskegee University to supply Salk and other labs with HeLa cells.[28] Less than a year later, Salk's vaccine was ready for human trials.[29]
Virology
HeLa cells have been used in testing how parvovirus infects cells of humans, dogs, and cats.[30] These cells have also been used to study viruses such as the oropouche virus (OROV). OROV causes disruption of cells in culture; the cells start to degenerate shortly after they are infected, causing viral induction of apoptosis.[31] HeLa cells have been used to study expression of the papillomavirus E2 and apoptosis.[32] HeLa cells have also been used to study the ability of the canine distemper virus to induce apoptosis in cancer cell lines,[33] which could play an important role in developing treatments for tumor cells resistant to radiation and chemotherapy.[33]
HeLa cells have also been instrumental in the development of human papilloma virus (HPV) vaccines. In the 1980s, Harald zur Hausen found that Lacks's cells from the original biopsy contained HPV-18, which was later found to be the cause of the aggressive cancer that had killed her. His work in linking HPV with cervical cancer won him a Nobel Prize and led to the development of HPV vaccines, which are predicted to reduce the number of deaths from cervical cancer by 70%.[34]
Over the years, HeLa cells have been infected with various types of viruses, including HIV, Zika, mumps, and herpesviruses to test and develop new vaccines and drugs. Dr. Richard Axel discovered that the addition of the CD4 protein to HeLa cells enabled them to be infected with HIV, allowing the virus to be studied.[35] In 1979, scientists learned that the measles virus constantly mutates when it infects HeLa cells,[36] and in 2019 they found that Zika cannot multiply in HeLa cells.[37]
Cancer
HeLa cells have been used in a number of cancer studies, including those involving sex steroid hormones, such as estradiol and estrogen, and estrogen receptors, along with estrogen-like compounds, such as quercetin, which has cancer-reducing properties.[38] There have also been studies on HeLa cells, involving the effects of flavonoids and antioxidants with estradiol on cancer cell proliferation.
In 2011, HeLa cells were used in tests of novel heptamethine dyes IR-808 and other analogues, which are currently being explored for their unique uses in medical diagnostics, the individualized treatment of cancer patients with the aid of PDT, co-administration with other drugs, and irradiation.[39][40] HeLa cells have been used in research involving fullerenes to induce apoptosis as a part of photodynamic therapy, as well as in in vitro cancer research using cell lines.[41] HeLa cells have also been used to define cancer markers in RNA, and have been used to establish an RNAi Based Identification System and Interference of Specific Cancer Cells.[42]
In 2014, HeLa cells were shown to provide a viable cell line for tumor xenografts in C57BL/6 nude mice,[43] and were subsequently used to examine the in vivo effects of fluoxetine and cisplatin on cervical cancer.
Genetics
In 1953, a lab mistake involving mixing HeLa cells with the wrong liquid allowed researchers for the first time to see and count each chromosome clearly in the HeLa cells with which they were working. This accidental discovery led scientists Joe Hin Tjio and Albert Levan to develop better techniques for staining and counting chromosomes.[34] They were the first to show that humans have 23 pairs of chromosomes rather than 24, as was previously believed. This was important for the study of developmental disorders, such as Down syndrome, that involve the number of chromosomes.
In 1965, Henry Harris and John Watkins created the first human-animal hybrid by fusing HeLa cells with mouse embryo cells. This enabled advances in mapping genes to specific chromosomes, which would eventually lead to the Human Genome Project.[34]
Space microbiology
In the 1960s, HeLa cells were sent on the Soviet satellite Sputnik-6 and human space missions to determine the long term effects of space travel on living cells and tissues. Scientists discovered that HeLa cells divide more quickly in zero gravity.[44]
Analysis
Telomerase
The HeLa cell line was derived for use in cancer research. These cells proliferate abnormally rapidly, even compared with other cancer cells. Like many other cancer cells,[45] HeLa cells have an active version of telomerase during cell division,[46] which copies telomeres over and over again. This prevents the incremental shortening of telomeres that is implicated in aging and eventual cell death. In this way, the cells circumvent the Hayflick limit, which is the limited number of cell divisions that most normal cells can undergo before becoming senescent. This results in unlimited cell division and immortality.
Chromosome number
Horizontal gene transfer from human papillomavirus 18 (HPV18) to human cervical cells created the HeLa genome, which is different from Henrietta Lacks's genome in various ways, including the number of chromosomes. HeLa cells are rapidly dividing cancer cells, and the number of chromosomes varies during cancer formation and cell culture. The current estimate (excluding very tiny fragments) is a "hypertriploid chromosome number (3n+)", which means 76 to 80 total chromosomes (rather than the normal diploid number of 46) with 22–25 clonally abnormal chromosomes, known as "HeLa signature chromosomes".[47][48][49][50] The signature chromosomes can be derived from multiple original chromosomes, making summary counts based on original numbering challenging. Researchers have also noted how stable these aberrant karyotypes can be.[47] Studies that combined spectral karyotyping, FISH, and conventional cytogenic techniques have shown that the detected chromosomal aberrations may be representative of advanced cervical carcinomas and were probably present in the primary tumor, since the HeLa genome has remained stable, even after years of continued cultivation.[47]
Complete genome sequence
The complete genome of HeLa cells was sequenced and published on 11 March 2013,[51][52] without the Lacks family's knowledge.[53] Concerns were raised by the family, so the authors voluntarily withheld access to the sequence data.[53] Jay Shendure led a HeLa sequencing project at the University of Washington, which resulted in a paper that had been accepted for publication in March 2013 – but that was also put on hold while the Lacks family's privacy concerns were addressed.[54] On 7 August 2013, NIH director Francis Collins announced a policy of controlled access to the cell line genome, based on an agreement reached after three meetings with the Lacks family.[55] A data-access committee will review requests from researchers for access to the genome sequence, under the criteria that the study is for medical research and that the users will abide by terms in the HeLa Genome Data Use Agreement, which includes that all NIH-funded researchers will deposit the data in a single database for future sharing. The committee consists of six members, including representatives from the medical, scientific, and bioethics fields, as well as two members of the Lacks family.[55] In an interview, Collins praised the Lacks family's willingness to participate in a situation that was thrust upon them. He described the whole experience with them as "powerful," saying that it brought together "science, scientific history and ethical concerns" in a unique way.[56]
Contamination
HeLa cells are sometimes difficult to control, because they adapt to growth in tissue culture plates and invade and outcompete other cell lines. Through improper maintenance, they have been known to contaminate other cell cultures in the same laboratory, interfering with biological research and forcing researchers to declare many results invalid. The degree of HeLa cell contamination among other cell types is unknown, because few researchers test the identity or purity of already established cell lines. It has been shown that a substantial fraction of in vitro cell lines are contaminated with HeLa cells; estimates range from 10% to 20%. This observation suggests that any cell line may be susceptible to a degree of contamination. Stanley Gartler (1967) and Walter Nelson-Rees (1975) were the first to publish on contamination of various cell lines by HeLa cells.[27] Gartler noted that "with the continued expansion of cell culture technology, it is almost certain that both interspecific and intraspecific contamination will occur."[9]
HeLa cell contamination has become a pervasive worldwide problem – affecting even the laboratories of many notable physicians, scientists, and researchers, including Jonas Salk. The HeLa contamination problem also contributed to Cold War tensions. The USSR and the USA had begun to cooperate in the war on cancer launched by President Richard Nixon, only to find that the exchanged cells were contaminated by HeLa.[57]
Rather than focus on how to resolve the problem of HeLa cell contamination, many scientists and science writers continue to document this problem as simply a contamination issue – caused not by human error or shortcomings but by the hardiness, proliferation, or overpowering nature of HeLa cells.[58] Recent data suggest that cross-contamination is still a major problem with modern cell cultures.[3][59] The International Cell Line Authentication Committee (ICLAC) notes that many cases of cell line misidentification are the result of cross-contamination of the culture by another, faster-growing cell line. This calls into question the validity of the research done using contaminated cell lines, as certain attributes of the contaminant, which may come from an entirely different species or tissue, may be misattributed to the cell line under investigation.[60]
New species proposal
HeLa cells were described by evolutionary biologist Leigh Van Valen as an example of the contemporary creation of a new species, dubbed Helacyton gartleri, owing to their ability to replicate indefinitely and their non-human number of chromosomes. The species was named after geneticist Stanley M. Gartler, whom Van Valen credits with discovering "the remarkable success of this species".[61] His argument for speciation depends on these points:
- the chromosomal incompatibility of HeLa cells with human cells;
- the ecological niche of HeLa cells;
- their ability to persist and expand well beyond the desires of human cultivators;
- the possession by HeLa cells of their own clonal karyotype, defining it as a distinct species.[62]
Van Valen proposed the new family Helacytidae and the genus Helacyton, and in the same paper proposed a new species for HeLa cells.[63]
However, this proposal was not taken seriously by other prominent evolutionary biologists, nor by scientists in other disciplines. Van Valen's argument that HeLa are a new species does not fulfill the criteria for an independent unicellular asexually reproducing species, because of the notorious instability of HeLa's karyotype and their lack of a strict ancestral-descendant lineage.[64]
Gallery
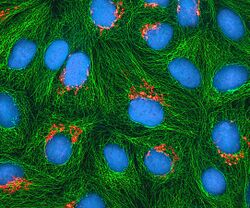
HeLa cells stained with Hoechst 33258
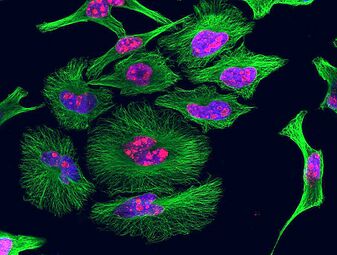
HeLa cells grown in culture and stained with antibody to tubulin (green), antibody to Ki-67 (red), and the blue DNA binding dye DAPI. The tubulin antibody shows the distribution of microtubules and the Ki-67 antibody is expressed in cells about to divide. Preparation, antibodies and image courtesy of EnCor Biotechnology.
A volumetric surface render (red) of the nuclear envelope of one HeLa cell. The cell was observed in 300 slices on electron microscopy and the nuclear envelope was automatically segmented and rendered. One vertical and one horizontal slice are added for reference.
Plasma membrane and nuclear envelope of one Hela cell, displayed as a volumetric surface rendering. Left and centre: the plasma membrane in blue, with transparency, and the nuclear envelope in solid cyan. Right: the plasma membrane without transparency and the same angle of view as the centre picture. The membranes have been segmented from data acquired by electron microscopy.
In media
- The 1997 documentary The Way of All Flesh by Adam Curtis explained the history of HeLa cells and their implications for medicine and society.[65]
- A 2010 episode of Law & Order, "Immortal", was heavily based on the story of Henrietta Lacks and the HeLa cell line, using the fictional "NaRo" cells as a stand-in.[66]
- The story of how the HeLa cell line came to be was also the subject of a 2010 episode of the podcast Radiolab.[67]
- HeLa cells were the subject of a 2010 book by Rebecca Skloot, The Immortal Life of Henrietta Lacks, investigating the historical context of the cell line and how the Lacks family was involved in its use.[13]
- A 2017 HBO film, The Immortal Life of Henrietta Lacks, was based on the book. The film starred Oprah Winfrey, Sylvia Grace Crim, and Rocky Carroll, with Renee Elise Goldsberry as Henrietta Lacks. Author Rebecca Skloot also appeared as a character in the film, portrayed by Rose Byrne.[68]
See also
- Clonally transmissible cancer
- Moore v. Regents of the University of California, case that set precedent for discarded tissue
- List of contaminated cell lines
- WI-38
References
- ↑ "A novel L1 retrotransposon marker for HeLa cell line identification". BioTechniques 46 (4): 277–284. 2009. doi:10.2144/000113089. PMID 19450234.
- ↑ Morris, Rhys Bowen (2023-08-02). "What were the top 100 cell lines of 2022?" (in en). https://blog.citeab.com/what-were-the-top-100-cell-lines-of-2022/.
- ↑ 3.0 3.1 "Check your cultures! A list of cross-contaminated or misidentified cell lines". Int. J. Cancer 127 (1): 1–8. 2010. doi:10.1002/ijc.25242. PMID 20143388.
- ↑ 4.0 4.1 Batts DW (2010-05-10). "Cancer cells killed Henrietta Lacks – then made her immortal". The Virginian-Pilot. pp. 1, 12–14. http://hamptonroads.com/2010/05/cancer-cells-killed-henrietta-lacks-then-made-her-immortal.
- ↑ 5.0 5.1 Scherer, W.F.; Syverton, J.T.; Gey, G.O. (1953). "Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix". Journal of Experimental Medicine 97 (5): 695–710. doi:10.1084/jem.97.5.695. PMID 13052828.
- ↑ "Johns Hopkins Magazine -- April 2000". https://pages.jh.edu/jhumag/0400web/01.html.
- ↑ "How One Woman's Cells Changed Medicine". ABC World News. 2010-01-31. https://abcnews.go.com/WN/womans-cells-changed-medicine/story?id=9712579.
- ↑ McKie, Robin (2010-04-03). "Henrietta Lacks's cells were priceless, but her family can't afford a hospital". The Guardian (London). http://theguardian.com/world/2010/apr/04/henrietta-lacks-cancer-cells.
- ↑ 9.0 9.1 9.2 9.3 Lucey, Brendan P.; Nelson-Rees, Walter A.; Hutchins, Grover M. (2009-10-21). "Henrietta Lacks, HeLa Cells, and Cell Culture Contamination" (in EN). Archives of Pathology & Laboratory Medicine 133 (9): 1463–7. doi:10.5858/133.9.1463. PMID 19722756. https://www.archivesofpathology.org/doi/10.1043/1543-2165-133.9.1463.
- ↑ Jones, Howard W. (1997-06-01). "Record of the first physician to see Henrietta Lacks at the Johns Hopkins Hospital: History of the beginning of the HeLa cell line" (in en). American Journal of Obstetrics and Gynecology 176 (6): s227–s228. doi:10.1016/S0002-9378(97)70379-X. ISSN 0002-9378. PMID 9215212. https://www.sciencedirect.com/science/article/pii/S000293789770379X.
- ↑ Butanis, Benjamin. "The Legacy of Henrietta Lacks" (in en). https://www.hopkinsmedicine.org/henriettalacks/.
- ↑ Washington, Harriet "Henrietta Lacks: An Unsung Hero", Emerge Magazine, October 1994
- ↑ 13.0 13.1 13.2 13.3 Zielinski, Sarah (2010-01-02). "Cracking the code of the human genome – Henrietta Lacks' 'immortal' cells". Smithsonian. https://www.smithsonianmag.com/science-nature/henrietta-lacks-immortal-cells-6421299/.
- ↑ White, Tracie (May 2, 2018), "Descendants of Henrietta Lacks discuss her famous cell line", Stanford Medicine News Center, https://med.stanford.edu/news/all-news/2018/05/descendants-of-henrietta-lacks-discuss-her-famous-cell-line.html, retrieved 2021-12-09
- ↑ del Carpio, Alexandra (April 27, 2014). "The Good, the Bad, and the HeLa". Berkeley Science Review. http://berkeleysciencereview.com/article/good-bad-hela/.
- ↑ Sharrer T (2006). ""HeLa" Herself". The Scientist 20 (7): 22. http://www.the-scientist.com/?articles.view/articleNo/24114/title/-HeLa--Herself/.
- ↑ Stump, Jessica L. (2014). "Henrietta Lacks and The HeLa Cell: Rights of Patients and Responsibilities of Medical Researchers". The History Teacher 48 (1): 127–180. ISSN 0018-2745.
- ↑ Day, Jo Ann. "Upholding the Highest Bioethical Standards | Johns Hopkins Medicine" (in en). https://www.hopkinsmedicine.org/henriettalacks/upholding-the-highest-bioethical-standards.html.
- ↑ 19.0 19.1 19.2 Skloot, Rebecca (2010). The Immortal Life of Henrietta Lacks. New York: Crown/Random House. ISBN 978-1-4000-5217-2.
- ↑ "HeLa Cell Line Origins, Contamination, Controversy, and Cytogenetics." (in en-US). https://www.hela-transfection.com/.
- ↑ "Estate of Henrietta Lacks sues Thermo Fisher over the improper sale of her immortal cells". https://endpts.com/estate-of-henrietta-lacks-sues-thermo-fisher-over-the-improper-sale-of-her-immortal-cells/.
- ↑ "Family of Henrietta Lacks hires civil rights attorney to seek funds over famous cells" (in en-US). Washington Post. ISSN 0190-8286. https://www.washingtonpost.com/local/henrietta-lacks-cells-family-compensation/2021/07/31/63935928-f16c-11eb-bf80-e3877d9c5f06_story.html.
- ↑ Holpuch, Amanda (1 August 2023). "Family of Henrietta Lacks Settles With Biotech Company That Used Her Cells". The New York Times. https://www.nytimes.com/2023/08/01/science/henrietta-lacks-cells-lawsuit-settlement.html.
- ↑ "Ultragenyx Sued by Henrietta Lacks' Family in Second HeLa Cell Line Lawsuit" (in en-US). https://www.biospace.com/article/ultragenyx-hela-lawsuit-placeholder/.
- ↑ Puck, T.T.; Marcus, P.I. (1955). "A rapid method for viable cell titration and clone production with Hela cells in tissue culture: The use of X-irradiated cells to supply conditioning factors". Proceedings of the National Academy of Sciences of the United States of America 41 (7): 432–437. July 15, 1955. doi:10.1073/pnas.41.7.432. PMID 16589695. Bibcode: 1955PNAS...41..432P.
- ↑ Smith, Van (April 17, 2002). "Wonder Woman: The Life, Death, and Life After Death of Henrietta Lacks, Unwitting Heroine of Modern Medical Science". Baltimore City Paper. http://www.citypaper.com/news/story.asp?id=3426.
- ↑ 27.0 27.1 Masters, John R. (2002). "HeLa cells 50 years on: The good, the bad and the ugly". Nature Reviews Cancer 2 (4): 315–319. doi:10.1038/nrc775. PMID 12001993.
- ↑ Turner, Timothy (2012). "Development of the Polio Vaccine: A Historical Perspective of Tuskegee University's Role in Mass Production and Distribution of HeLa Cells". Journal of Health Care for the Poor and Underserved 23 (4a): 5–10. doi:10.1353/hpu.2012.0151. PMID 23124495.
- ↑ Brownlee, K. A. (1955). "Statistics of the 1954 Polio Vaccine Trials*". Journal of the American Statistical Association 50 (272): 1005–1013. doi:10.1080/01621459.1955.10501286.
- ↑ Parker, J; Murphy W; Wang D; O'Brien S; Parrish C (2001). "Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells". Journal of Virology 75 (8): 3896–3902. doi:10.1128/JVI.75.8.3896-3902.2001. PMID 11264378.
- ↑ Acrani, G.O.; Gomes, R.; Proença-Módena, J.L.; da Silva, A.F.; Carminati, P.O.; Silva, M.L.; Santos, R.I.; Arruda, E. (2010). "Apoptosis induced by Oropouche virus infection in HeLa cells is dependent on virus protein expression". Virus Research 149 (1): 56–63. doi:10.1016/j.virusres.2009.12.013. PMID 20080135.
- ↑ Hou, S.Y.; Wu, S.; Chiang, C. (2002). "Transcriptional activity among high and low risk human papillomavirus E2 proteins correlates with E2 DNA binding". The Journal of Biological Chemistry 277 (47): 45619–45629. doi:10.1074/jbc.M206829200. PMID 12239214.
- ↑ 33.0 33.1 del Puerto, H.L.; Martins, A.S.; Milsted, A.; Souza-Fagundes, E.M.; Braz, G.F.; Hissa, B.; Andrade, L.O.; Alves, F. et al. (2011). "Canine distemper virus induces apoptosis in cervical tumor derived cell lines". Virol. J. 8 (1): 334. doi:10.1186/1743-422X-8-334. PMID 21718481.
- ↑ 34.0 34.1 34.2 MacDonald, Anna (13 June 2018). "5 Contributions HeLa Cells Have Made to Science" (in en). https://www.technologynetworks.com/cell-science/lists/5-contributions-hela-cells-have-made-to-science-305036.
- ↑ Mondor, Isabelle; Ugolini, Sophie; Sattentau, Quentin J. (1998-05-01). "Human Immunodeficiency Virus Type 1 Attachment to HeLa CD4 Cells Is CD4 Independent and gp120 Dependent and Requires Cell Surface Heparans". Journal of Virology 72 (5): 3623–3634. doi:10.1128/jvi.72.5.3623-3634.1998. ISSN 1098-5514. PMID 9557643.
- ↑ Wechsler, Steven L.; Rustigian, Robert; Stallcup, Kathryn C.; Byers, Karen B.; Winston, Stuart H.; Fields, Bernard N. (1979). "Measles Virus-Specified Polypeptide Synthesis in Two Persistently Infected HeLa Cell Lines". Journal of Virology 31 (3): 677–684. doi:10.1128/jvi.31.3.677-684.1979. ISSN 0022-538X. PMID 513191.
- ↑ Li, Li; Collins, Natalie D.; Widen, Steven G.; Davis, Emily H.; Kaiser, Jaclyn A.; White, Mellodee M.; Greenberg, M. Banks; Barrett, Alan D. T. et al. (2019-08-20). "Attenuation of Zika Virus by Passage in Human HeLa Cells". Vaccines 7 (3): 93. doi:10.3390/vaccines7030093. ISSN 2076-393X. PMID 31434319.
- ↑ Pamela Bulzomi; Paola Galluzzo; Alessandro Bolli; Stefano Leone; Filippo Acconcia; Maria Marino (2012). "The pro-apoptotic effect of quercetin in cancer cell lines requires ERβ-dependent signals". Journal of Cellular Physiology 227 (5): 1891–1898. doi:10.1002/jcp.22917. PMID 21732360.
- ↑ "A NIR heptamethine Dye with intrinsic cancer targeting, imaging and photosynthesizing properties". Journal of Biomaterials China 33 (7): 2230–2239. 2011. doi:10.1016/j.biomaterials.2011.11.081. PMID 22182749.
- ↑ Pene, F.; Courtine, E.; Cariou, A.; Mira, J.P. (2009). "Toward theranostics". Crit Care Med 37 (1 Suppl): S50–S58. doi:10.1097/CCM.0b013e3181921349. PMID 19104225.
- ↑ Briiuner., Thomas; Dieter F. Hulser (1990). "Tumor Cell Invasion and Gap Junctional Communication". Invasion Metastasis 10: 31–34. http://elib.uni-stuttgart.de/opus/volltexte/2011/6882/pdf/huel63.pdf. Retrieved 3 April 2012.
- ↑ Xie, Z.; Wroblewska, L.; Prochazka, L.; Weiss, R.; Benenson, Y. (2011). "Multi-Input RNAi-Based Logic Circuit for Identification of Specific Cancer Cells". Science 333 (6047): 1307–1311. doi:10.1126/science.1205527. PMID 21885784. Bibcode: 2011Sci...333.1307X. http://courses.cs.washington.edu/courses/cse599x/10sp/RNAi_circuits.pdf.
- ↑ Arjomandnejad, M (2014). "HeLa cell line xenograft tumor as a suitable cervical cancer model: growth kinetic characterization and immunohistochemistry array". Archives of Iranian Medicine 17 (4): 273–277. PMID 24724604. https://pdfs.semanticscholar.org/4584/d1d5efb89d7ab1001a96ba5f7d2a69596cfe.pdf.
- ↑ Davies, Preston. "Significant Research Advances Enabled by HeLa Cells" (in en-US). https://osp.od.nih.gov/scientific-sharing/hela-cells-timeline/.
- ↑ The Nobel Prize in Physiology or Medicine 2009 on nobelprize.org
- ↑ "Telomerase activity in HeLa cervical carcinoma cell line proliferation". Biogerontology 8 (2): 163–72. 2007. doi:10.1007/s10522-006-9043-9. PMID 16955216.
- ↑ 47.0 47.1 47.2 "Comprehensive and definitive molecular cytogenetic characterization of HeLa cells by spectral karyotyping". Cancer Res. 59 (1): 141–50. 1999. PMID 9892199.
- ↑ "The genomic and transcriptomic landscape of a HeLa cell line". G3: Genes, Genomes, Genetics 3 (8): 1213–24. 2013. doi:10.1534/g3.113.005777. PMID 23550136.
- ↑ Bottomley, R.H.; Trainer, A.L.; Griffin, M.J. (1969). "Enzymatic and chromosomal characterization of HeLa variants". J. Cell Biol. 41 (3): 806–15. doi:10.1083/jcb.41.3.806. PMID 5768876.
- ↑ Andrew Adey; Joshua N. Burton; Jacob O. Kitzman; Joseph B. Hiatt; Alexandra P. Lewis; Beth K. Martin; Ruolan Qiu; Choli Lee et al. (2013). "The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line". Nature 500 (7461): 207–211. August 8, 2013. doi:10.1038/nature12064. PMID 23925245. Bibcode: 2013Natur.500..207A.
- ↑ "The genomic and transcriptomic landscape of a HeLa cell line". G3: Genes, Genomes, Genetics 3 (8): 1213–24. 2013. doi:10.1534/g3.113.005777. PMID 23550136.
- ↑ Callaway, Ewen (March 15, 2013). "Most popular human cell in science gets sequenced". Nature. doi:10.1038/nature.2013.12609. http://www.nature.com/news/most-popular-human-cell-in-science-gets-sequenced-1.12609. Retrieved 8 August 2013.
- ↑ 53.0 53.1 Callaway, Ewen (March 27, 2013). "HeLa publication brews bioethical storm". Nature. doi:10.1038/nature.2013.12689. http://www.nature.com/news/hela-publication-brews-bioethical-storm-1.12689. Retrieved 8 August 2013.
- ↑ Callaway, Ewen (August 7, 2013). "Deal done over HeLa cell line". Nature 500 (7461): 132–133. doi:10.1038/500132a. PMID 23925220. Bibcode: 2013Natur.500..132C.
- ↑ 55.0 55.1 "NIH, Lacks family reach understanding to share genomic data of HeLa cells". The National Institutes of Health. August 7, 2013. http://nih.gov/news/health/aug2013/nih-07.htm.
- ↑ Callaway, Ewen (August 7, 2013). "NIH director explains HeLa agreement". Nature. doi:10.1038/nature.2013.13521.
- ↑ Gold, Michael (1986). A conspiracy of cells: one woman's immortal legacy and the medical scandal it caused. State University of New York Press. ISBN 0-88706-099-4. OCLC 12805138.
- ↑ "Comparative analysis and integrative classification of NCI60 cell lines and primary tumors using gene expression profiling data". BMC Genomics 7 (1): 166. 2006. doi:10.1186/1471-2164-7-166. PMID 16817967.
- ↑ Nardone, R.M. (2007). "Eradication of cross-contaminated cell lines: A call for action". Cell Biology and Toxicology 23 (6): 367–372. doi:10.1007/s10565-007-9019-9. PMID 17522957. http://www.sivb.org/publicPolicy_Eradication.pdf. Retrieved 2007-12-04.
- ↑ "ATCC® Standards Development Organization: The International Cell Line Authentication Committee (ICLAC)". Standards.atcc.org. http://standards.atcc.org/kwspub/home/the_international_cell_line_authentication_committee-iclac_/.
- ↑ "Hela, a new microbial species" (in en). Evolutionary Theory 10 (2): 71–4. 7 February 1991. http://www.mn.uio.no/cees/english/services/evolutionary-theory/volume-10/vol-10-no-2-pages-71-74-l-van-valen-and-v-c-mairorana-hela-a-new-microbial-species.pdf.
- ↑ Duesberg, P; Mandrioli, D; McCormack, A; Nicholson, JM (2011). "Is carcinogenesis a form of speciation?". Cell Cycle (Georgetown, Texas) 10 (13): 2100–2114. doi:10.4161/cc.10.13.16352. PMID 21666415.
- ↑ van Valen, L.M.; Maiorana, V.C. (1991). "HeLa, a new microbial species". Evolutionary Theory & Review 10: 71–74. ISSN 1528-2619.
- ↑ Cohan, FM (2002). "What are bacterial species?". Annu. Rev. Microbiol. 56 (1): 457–487. doi:10.1146/annurev.micro.56.012302.160634. PMID 12142474. http://wesscholar.wesleyan.edu/cgi/viewcontent.cgi?article=1366&context=div3facpubs.
- ↑ "Modern Times: The Way of All Flesh - BBC Two England - 19 March 1997". The Radio Times (3815): 92. 1997-03-13. http://genome.ch.bbc.co.uk/efaa1adefb274189a158fc5676627d32.
- ↑ Thomas, June (2010-05-19). "Ripped From Which Headline? "Immortal"" (in en). https://slate.com/culture/2010/05/ripped-from-which-headline-immortal.html.
- ↑ "Henrietta's Tumor". https://www.wnycstudios.org/podcasts/radiolab/segments/91716-henriettas-tumor.
- ↑ "About The Immortal Life of Henrietta Lacks". Rebecca Skloot. http://rebeccaskloot.com/the-immortal-life/.
Further reading
- Hannah Landecker (2000). "Immortality, In Vitro: A History of the HeLa Cell Line". in Brodwin, Paul. Biotechnology and culture: bodies, anxieties, ethics. Bloomington: Indiana University Press. pp. 53–74. ISBN 978-0-253-21428-7. https://archive.org/details/biotechnologycul0000unse/page/53.
- Rebecca Skloot (2010). The Immortal Life of Henrietta Lacks.
External links
- HeLa (CCL-2 Cells) in the ATCC database
- HeLa Cells at the US National Library of Medicine Medical Subject Headings (MeSH)
- HeLa Transfection and Selection Data for HeLa Cells
- Rebecca Skloot, The Immortal Life of Henrietta Lacks book website with additional features (photo/video/audio)
- The Henrietta Lacks Foundation, a foundation established to, among other things, help provide scholarship funds and health insurance to Henrietta Lacks's family.
- "Wonder Woman: The Life, Death, and Life After Death of Henrietta Lacks, Unwitting Heroine of Modern Medical Science" by Van Smith
- "What's Left of Henrietta Lacks?" by Anne Enright
- Cell Centered Database – HeLa cell
- Cellosaurus entry for HeLa
- The Legacy of Henrietta Lacks
 |