Chemistry:Thiophosgene
|
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Carbonothioyl dichloride
| |||
| Other names
Thiophosgene; Thiocarbonyl chloride; Carbonothioic dichloride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| CSCl 2 | |||
| Molar mass | 114.97 g·mol−1 | ||
| Appearance | Red liquid | ||
| Odor | Persistent, choking odor | ||
| Density | 1.50 g/cm3 | ||
| Boiling point | 70 to 75 °C (158 to 167 °F; 343 to 348 K) | ||
| Decomposes | |||
| Solubility in other solvents | Reacts with amines and alcohols, soluble in polar organic solvents | ||
| -50.6·10−6 cm3/mol | |||
Refractive index (nD)
|
1.558 | ||
| Structure | |||
| planar, sp2, C2v | |||
| Hazards | |||
| Main hazards | Highly toxic | ||
| Flash point | 62 °C (144 °F; 335 K) | ||
| Related compounds | |||
Related compounds
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
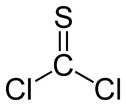
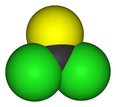
Thiophosgene is a red liquid with the formula CSCl
2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.[1]
Preparation
CSCl
2 is prepared in a two-step process from carbon disulfide. In the first step, carbon disulfide is chlorinated to give trichloromethanesulfenyl chloride (perchloromethyl mercaptan), CCl
3SCl:
- CS
2 + 3 Cl
2 → CCl
3SCl + S
2Cl
2
The chlorination must be controlled as excess chlorine converts trichloromethanesulfenyl chloride into carbon tetrachloride. Steam distillation separates the trichloromethanesulfenyl chloride, a rare sulfenyl chloride, and hydrolyzes the disulfur dichloride. Reduction of trichloromethanesulfenyl chloride produces thiophosgene:
- CCl
3SCl + M → CSCl
2 + MCl
2
Tin[2] and dihydroanthracene[3] have been used for the reducing agents.
Reactions
CSCl
2 is mainly used to prepare compounds with the connectivity CSX
2 where X = OR, NHR.[4] Such reactions proceed via intermediate such as CSClX. Under certain conditions, one can convert primary amines into isothiocyanates.
CSCl
2 also serves as a dienophile to give, after reduction 5-thiacyclohexene derivatives. Thiophosgene is also known as the appropriate reagent in Corey-Winter synthesis for stereospecific conversion of 1,2-diols into alkenes.[5]
It forms a head-to-tail dimer upon irradiation with UV light:[6]
- 2 CSCl
2 → S
2(CCl
2)
2
Unlike thiophosgene monomer, a red liquid, the photodimer, an example of a 1,3-dithietane, is a colourless solid.
Thisphosgene decomposes at 200 °C or above to form carbon disulfide and carbon tetrachloride.[7] It has also been observed decomposing to hydrogen sulfide, hydrogen chloride, and carbonyl sulfide gases via contact with human tissue.[8]
Toxicology and safety
CSCl
2 is considered highly toxic. Inhalation of the substance can cause irritation of respiratory system, burns, delayed pulmonary edema and death. [9]
See also
References
- ↑ Manchiu D. S. Lay, Mitchell W. Sauerhoff And Donald R. Saunders "Carbon Disulfide" in Ullmann's Encyclopedia Of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a05_185
- ↑ Dyson, G. M. (1926). "Thiophosgene". Organic Syntheses 6: 86. doi:10.15227/orgsyn.006.0086.
- ↑ K. T. Potts, C. Sapino (1972). "Thiocarbonyl halides". in S. Patai. Acyl Halides. PATAI'S Chemistry of Functional Groups. pp. 349–380. doi:10.1002/9780470771273.ch11. ISBN 978-0-470-77127-3.
- ↑ Pascual, Roxana Martinez "Thiophosgene" Synlett 2015, vol. 26, pp. 1776-1777.doi:10.1055/s-0034-1380659
- ↑ Sharma, S. (1978). "Thiophosgene in Organic Synthesis". Synthesis 1978 (11): 803–820. doi:10.1055/s-1978-24896.
- ↑ B. Krebs H. Beyer (1969). "Die Kristall‐ und Molekelstruktur des dimeren Thiophosgens". Z. Anorg. Allg. Chem. 365 (3–4): 199–210. doi:10.1002/zaac.19693650315.
- ↑ "Thiophosgene". https://pubchem.ncbi.nlm.nih.gov/compound/Thiophosgene.
- ↑ "Thiophosgene". https://pubchem.ncbi.nlm.nih.gov/compound/Thiophosgene.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs named{{{1}}}
Further reading
 |