Chemistry:Bexagliflozin
 | |
| Clinical data | |
|---|---|
| Trade names | Brenzavvy, Bexacat |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
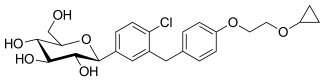
| Formula | C24H29ClO7 |
| Molar mass | 464.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Bexagliflozin, sold under the brand name Brenzavvy, is an antidiabetic medication used to improve glycemic control in adults with type 2 diabetes as an adjunct to diet and exercise.[4] It is a sodium-glucose cotransporter 2 (SGLT2) inhibitor that is taken by mouth.[1][2]
Medical uses
Bexagliflozin is indicated to improve glycemic control in adults with type 2 diabetes as an adjunct to diet and exercise.[3][4]
Research
A 96-week phase II clinical study of adults with type 2 diabetes showed that bexagliflozin monotherapy provided a durable, clinically meaningful improvement of glycemic control, with a substantial reduction in weight and blood pressure, but no increase in the rate of significant adverse events.[5][6] In a clinical study of patients with type 2 diabetes and stage 3a/3b chronic kidney disease, bexagliflozin was well tolerated and shown to reduce hemoglobin A1c levels, body weight, systolic blood pressure and albuminuria.[7]
Veterinary uses
The data from two six-month field studies and an extended use field study demonstrated that bexagliflozin was over 80% effective in improving glycemic control in cats with diabetes mellitus.[2]
Bexagliflozin, sold under the brand name Bexacat, is an antidiabetic medication used to improve glycemic control in cats with diabetes.[2] Bexacat is the first sodium-glucose cotransporter 2 (SGLT2) inhibitor new animal drug approved by the US Food and Drug Administration (FDA) in any animal species.[2] It was approved for medical use in the United States in December 2022.[2][8] Bexacat is sponsored by Increvet Inc., based in Boston, Massachusetts.[2] Elanco licensed development and commercialization rights for bexagliflozin from Bexcafe, an affiliate of Increvet.[8]
References
- ↑ 1.0 1.1 "Bexacat- bexagliflozin tablets tablet". 16 January 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f918583d-0337-40da-8da1-1e1320b8d027.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "FDA Approves First Oral Treatment for Cats with Diabetes Mellitus". U.S. Food and Drug Administration (FDA). 8 December 2022. https://www.fda.gov/animal-veterinary/cvm-updates/fda-approves-first-oral-treatment-cats-diabetes-mellitus.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 3.0 3.1 "Brenzavvy- bexagliflozin tablet". 18 September 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3cdf28fc-4194-4ad6-aa03-c9eaa68da83e.
- ↑ 4.0 4.1 "Novel Drug Approvals for 2023". 20 January 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "A 96-week, double-blind, randomized controlled trial comparing bexagliflozin to glimepiride as an adjunct to metformin for the treatment of type 2 diabetes in adults". Diabetes, Obesity and Metabolism 25 (1): 293–301. September 2022. doi:10.1111/dom.14875. PMID 36178197.
- ↑ "A 96-week, multinational, randomized, double-blind, parallel-group, clinical trial evaluating the safety and effectiveness of bexagliflozin as a monotherapy for adults with type 2 diabetes". Diabetes, Obesity and Metabolism 21 (11): 2496–2504. November 2019. doi:10.1111/dom.13833. PMID 31297965.
- ↑ "Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD". American Journal of Kidney Diseases 74 (3): 328–337. September 2019. doi:10.1053/j.ajkd.2019.03.417. PMID 31101403.
- ↑ 8.0 8.1 "Elanco Announces FDA Approval of Bexacat (bexagliflozin tablets) – the First-of-its-Kind Oral Feline Diabetes Treatment Option" (Press release). Elanco. 9 December 2022. Archived from the original on 11 December 2022. Retrieved 11 December 2022.
Further reading
- "Evaluation of bexagliflozin in cats with poorly regulated diabetes mellitus". Canadian Journal of Veterinary Research 86 (1): 52–58. January 2022. PMID 34975223.
- "Metabolism and disposition of the SGLT2 inhibitor bexagliflozin in rats, monkeys and humans". Xenobiotica 50 (5): 559–569. May 2020. doi:10.1080/00498254.2019.1654634. PMID 31432741.
 |

