Chemistry:Danuglipron
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | PF-06882961 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
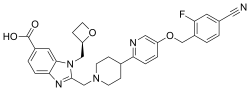
| Formula | C31H30FN5O4 |
| Molar mass | 555.610 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Danuglipron is a small-molecule GLP-1 agonist developed by Pfizer[1] that, in an oral formulation, is under investigation as a therapy for diabetes mellitus. Initial results from a randomized controlled trial indicate that it reduced weight and improved diabetic control. The most commonly reported adverse events were nausea, diarrhea, and vomiting.[2][3]
See also
- Lotiglipron
- Orforglipron
References
- ↑ "A Small-Molecule Oral Agonist of the Human Glucagon-like Peptide-1 Receptor". Journal of Medicinal Chemistry 65 (12): 8208–8226. June 2022. doi:10.1021/acs.jmedchem.1c01856. PMID 35647711.
- ↑ "Efficacy and Safety of Oral Small Molecule Glucagon-Like Peptide 1 Receptor Agonist Danuglipron for Glycemic Control Among Patients With Type 2 Diabetes: A Randomized Clinical Trial". JAMA Network Open 6 (5): e2314493. May 2023. doi:10.1001/jamanetworkopen.2023.14493. PMID 37213102.
- ↑ Constantino, Annika Kim (2023-12-02). "Pfizer's twice-daily weight loss pill joins a long list of obesity drug flops" (in en). https://www.cnbc.com/2023/12/02/pfizer-weight-loss-pill-joins-list-of-obesity-drug-flops.html.
 |

