Biology:PD-L1
 Generic protein structure example |
Programmed death-ligand 1 (PD-L1) also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1) is a protein that in humans is encoded by the CD274 gene.[1]
Programmed death-ligand 1 (PD-L1) is a 40kDa type 1 transmembrane protein that has been speculated to play a major role in suppressing the adaptive arm of immune systems during particular events such as pregnancy, tissue allografts, autoimmune disease and other disease states such as hepatitis. Normally the adaptive immune system reacts to antigens that are associated with immune system activation by exogenous or endogenous danger signals. In turn, clonal expansion of antigen-specific CD8+ T cells and/or CD4+ helper cells is propagated. The binding of PD-L1 to the inhibitory checkpoint molecule PD-1 transmits an inhibitory signal based on interaction with phosphatases (SHP-1 or SHP-2) via Immunoreceptor Tyrosine-Based Switch Motif (ITSM).[2] This reduces the proliferation of antigen-specific T-cells in lymph nodes, while simultaneously reducing apoptosis in regulatory T cells (anti-inflammatory, suppressive T cells) – further mediated by a lower regulation of the gene Bcl-2.[citation needed]
History
PD-L1 also known as B7-H1 was characterized at the Mayo Clinic in 1999 as an immune regulatory molecule.[3] At that time, it was concluded that B7-H1 helps tumor cells evade anti-tumor immunity.[4] In 2003, B7-H1 was shown to be expressed on myeloid cells as checkpoint protein and was proposed as potential target in cancer immunotherapy in human clinic.[5]
Binding
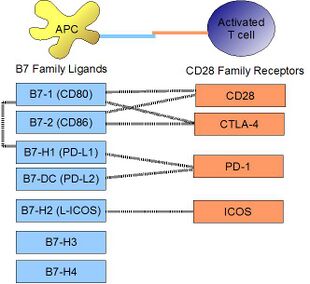
PD-L1 binds to its receptor, PD-1, found on activated T cells, B cells, and myeloid cells, to modulate activation or inhibition. The affinity between PD-L1 and PD-1, as defined by the dissociation constant Kd, is 770 nM. PD-L1 also has an appreciable affinity for the costimulatory molecule CD80 (B7-1), but not CD86 (B7-2).[6] CD80's affinity for PD-L1, 1.4 µM, is intermediate between its affinity for CD28 and CTLA-4 (4.0 µM and 400 nM, respectively). The related molecule PD-L2 has no such affinity for CD80 or CD86, but shares PD-1 as a receptor (with a stronger Kd of 140 nM). Said et al. showed that PD-1, up-regulated on activated CD4 T-cells, can bind to PD-L1 expressed on monocytes and induces IL-10 production by the latter.[7]
Signaling
Engagement of PD-L1 with its receptor PD-1 on T cells delivers a signal that inhibits TCR-mediated activation of IL-2 production and T cell proliferation. The mechanism involves inhibition of ZAP70 phosphorylation and its association with CD3ζ.[8] PD-1 signaling attenuates PKC-θ activation loop phosphorylation (resulting from TCR signaling), necessary for the activation of transcription factors NF-κB and AP-1, and for production of IL-2. PD-L1 binding to PD-1 also contributes to ligand-induced TCR down-modulation during antigen presentation to naive T cells, by inducing the up-regulation of the E3 ubiquitin ligase CBL-b.[9]
Regulation
By interferons
Upon IFN-γ stimulation, PD-L1 is expressed on T cells, NK cells, macrophages, myeloid DCs, B cells, epithelial cells, and vascular endothelial cells.[10] The PD-L1 gene promoter region has a response element to IRF-1, the interferon regulatory factor.[11] Type I interferons can also upregulate PD-L1 on murine hepatocytes, monocytes, DCs, and tumor cells.[12]
On macrophages and monocytes
PD-L1 is notably expressed on macrophages. In the mouse, it has been shown that classically activated macrophages (induced by type I helper T cells or a combination of LPS and interferon-gamma) greatly upregulate PD-L1.[13] Alternatively, macrophages activated by IL-4 (alternative macrophages), slightly upregulate PD-L1, while greatly upregulating PD-L2. It has been shown by STAT1-deficient knock-out mice that STAT1 is mostly responsible for upregulation of PD-L1 on macrophages by LPS or interferon-gamma, but is not at all responsible for its constitutive expression before activation in these mice. It was also shown that PD-L1 is constituvely expressed on mouse Ly6Clo nonclassical monocytes in steady state.[14]
Role of microRNAs
Resting human cholangiocytes express PD-L1 mRNA, but not the protein, due to translational suppression by microRNA miR-513.[15] Upon treatment with interferon-gamma, miR-513 was down-regulated, thereby lifting suppression of PD-L1 protein. In this way, interferon-gamma can induce PD-L1 protein expression by inhibiting gene-mediated suppression of mRNA translation. Whereas the Epstein-Barr viral (EBV) latent membrane protein-1 (LMP1) is a known potent inducer of PD-L1, the EBV miRNA miR-BamH1 fragment H rightward open reading frame 1 (BHRF1) 2-5p has been shown to regulate LMP1 induced PD-L1 expression.[16]
Epigenetic regulation
PD-L1 promoter DNA methylation may predict survival in some cancers after surgery.[17]
Clinical significance
Cancer
PD-L1 is shown to be highly expressed in a variety of malignancies, particularly lung cancer. In order to anticipate the effectiveness of gene therapy or systemic immunotherapy in blocking the PD-1 and PD-L1 checkpoints, PD-L1 might be employed as a prognostic marker and a target for anti-cancer immunity.[18] i.e. upregulation of PD-L1 may allow cancers to evade the host immune system. For example, an analysis of 196 tumor specimens from patients with renal cell carcinoma found that high tumor expression of PD-L1 was associated with increased tumor aggressiveness and a 4.5-fold increased risk of death.[19]
Many PD-L1 inhibitors are in development as immuno-oncology therapies and are showing good results in clinical trials.[20] Clinically available examples include durvalumab, atezolizumab and avelumab.[21] In normal tissue, feedback between transcription factors like STAT3 and NF-κB restricts the immune response to protect host tissue and limit inflammation. In cancer, loss of feedback restriction between transcription factors can lead to increased local PD-L1 expression, which could limit the effectiveness of systemic treatment with agents targeting PD-L1.[22] CAR-T[23] and NK cells[24] targeting PD-L1 are being evaluated for treating cancer. pSTAT-1 and PDL-1 expressions also strongly correlate in prostate cancer.[25]
Upregulation of PD-L1 on immune cells (especially myeloid cells) can also lead to formation of an immunosuppressive environment in a highly localized manner that also allow the cancer cells to proliferate.[26]
PD-L1 analysis in TNBC is essential for selecting patients eligible for immunotherapy. Inter-observer and intra-observer agreement among the pathologists were found to be substantial. Cases around the 1% cut-off value are specifically challenging.[27]
Listeria monocytogenes
In a mouse model of intracellular infection, L. monocytogenes induced PD-L1 protein expression in T cells, NK cells, and macrophages. PD-L1 blockade (using blocking antibodies) resulted in increased mortality for infected mice. Blockade reduced TNFα and nitric oxide production by macrophages, reduced granzyme B production by NK cells, and decreased proliferation of L. monocytogenes antigen-specific CD8 T cells (but not CD4 T cells).[28] This evidence suggests that PD-L1 acts as a positive costimulatory molecule in intracellular infection.
Autoimmunity
PD-1/PD-L1 interaction is thought to play a role in preventing destructive autoimmunity, especially during inflammatory conditions. The best example is in the stomach, where PD-1 expression protects the gastrin expressing G-cells from the immune system during Helicobacter pylori-provoked inflammation.[29] But also a variety of pre-clinical studies support the notion that the PD-1/PD-L1 interaction is implicated in autoimmunity. NOD mice, an animal model for autoimmunity that exhibit a susceptibility to spontaneous development of type I diabetes and other autoimmune diseases, have been shown to develop precipitated onset of diabetes from blockade of PD-1 or PD-L1 (but not PD-L2).[30]
In humans, PD-L1 was found to have altered expression in pediatric patients with systemic lupus erythematosus (SLE). Studying isolated PBMC from healthy children, immature myeloid dendritic cells and monocytes expressed little PD-L1 at initial isolation, but spontaneously up-regulated PD-L1 by 24 hours. In contrast, both mDC and monocytes from patients with active SLE failed to upregulate PD-L1 over a 5-day time course, expressing this protein only during disease remissions.[31] This may be one mechanism whereby peripheral tolerance is lost in SLE.
See also
References
- ↑ "Entrez Gene: CD274 CD274 molecule". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=29126.
- ↑ "SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation". Journal of Immunology 173 (2): 945–954. July 2004. doi:10.4049/jimmunol.173.2.945. PMID 15240681.
- ↑ "B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion". Nature Medicine 5 (12): 1365–1369. December 1999. doi:10.1038/70932. PMID 10581077.
- ↑ "Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion". Nature Medicine 8 (8): 793–800. August 2002. doi:10.1038/nm730. PMID 12091876.
- ↑ "Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity". Nature Medicine 9 (5): 562–567. May 2003. doi:10.1038/nm863. PMID 12704383.
- ↑ "Interaction of human PD-L1 and B7-1". Molecular Immunology 45 (13): 3567–3572. August 2008. doi:10.1016/j.molimm.2008.05.014. PMID 18585785.
- ↑ "Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection". Nature Medicine 16 (4): 452–459. April 2010. doi:10.1038/nm.2106. PMID 20208540.
- ↑ "PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta". FEBS Letters 574 (1–3): 37–41. September 2004. doi:10.1016/j.febslet.2004.07.083. PMID 15358536.
- ↑ "PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells". EMBO Molecular Medicine 3 (10): 581–592. October 2011. doi:10.1002/emmm.201100165. PMID 21739608.
- ↑ "The new B7s: playing a pivotal role in tumor immunity". Journal of Immunotherapy 30 (3): 251–260. April 2007. doi:10.1097/CJI.0b013e31802e085a. PMID 17414316.
- ↑ "Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274)". FEBS Letters 580 (3): 755–762. February 2006. doi:10.1016/j.febslet.2005.12.093. PMID 16413538.
- ↑ "Expression of programmed death 1 ligands by murine T cells and APC". Journal of Immunology 169 (10): 5538–5545. November 2002. doi:10.4049/jimmunol.169.10.5538. PMID 12421930.
- ↑ "PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells". Proceedings of the National Academy of Sciences of the United States of America 100 (9): 5336–5341. April 2003. doi:10.1073/pnas.0931259100. PMID 12697896. Bibcode: 2003PNAS..100.5336L.
- ↑ "PD-L1 expression on nonclassical monocytes reveals their origin and immunoregulatory function". Science Immunology 4 (36): eaar3054. June 2019. doi:10.1126/sciimmunol.aar3054. PMID 31227596.
- ↑ "MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes". Journal of Immunology 182 (3): 1325–1333. February 2009. doi:10.4049/jimmunol.182.3.1325. PMID 19155478.
- ↑ "EBV microRNA-BHRF1-2-5p targets the 3'UTR of immune checkpoint ligands PD-L1 and PD-L2". Blood 134 (25): 2261–2270. December 2019. doi:10.1182/blood.2019000889. PMID 31856276.
- ↑ "PD-L1 promoter methylation is a prognostic biomarker for biochemical recurrence-free survival in prostate cancer patients following radical prostatectomy". Oncotarget 7 (48): 79943–79955. November 2016. doi:10.18632/oncotarget.13161. PMID 27835597.
- ↑ "Soluble PD-L1 Expression After Intravenous Treatment of Cancer Patients With Selenite in Phase I Clinical Trial" (in English). Frontiers in Oncology 12: 906134. 2022-06-02. doi:10.3389/fonc.2022.906134. PMID 35720000.
- ↑ "Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target". Proceedings of the National Academy of Sciences of the United States of America 101 (49): 17174–17179. December 2004. doi:10.1073/pnas.0406351101. PMID 15569934. Bibcode: 2004PNAS..10117174T.
- ↑ "Programmed death ligand-1 expression in non-small cell lung cancer". Laboratory Investigation; A Journal of Technical Methods and Pathology 94 (1): 107–116. January 2014. doi:10.1038/labinvest.2013.130. PMID 24217091.
- ↑ "Immune checkpoint inhibitors to treat cancer". https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html.
- ↑ "Aberrant control of NF-κB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: molecular mode". Cancer Biology & Medicine 14 (3): 254–270. August 2017. doi:10.20892/j.issn.2095-3941.2017.0029. PMID 28884042.
- ↑ "Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice". Proceedings of the National Academy of Sciences of the United States of America 116 (16): 7624–7631. April 2019. doi:10.1073/pnas.1817147116. PMID 30936321. Bibcode: 2019PNAS..116.7624X.
- ↑ "PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations". Journal for Immunotherapy of Cancer 8 (1): e000450. May 2020. doi:10.1136/jitc-2019-000450. PMID 32439799.
- ↑ "The association between PI3K, JAK/STAT pathways with the PDL-1 expression in prostate cancer". Andrologia 54 (e14541): e14541. July 2022. doi:10.1111/and.14541. PMID 35880672.
- ↑ "The Spatial Landscape of Progression and Immunoediting in Primary Melanoma at Single-Cell Resolution". Cancer Discovery 12 (6): 1518–1541. June 2022. doi:10.1158/2159-8290.CD-21-1357. PMID 35404441.
- ↑ Zaakouk, M.; Van Bockstal, M.; Galant, C.; Callagy, G.; Provenzano, E.; Hunt, R.; D’Arrigo, C.; Badr, N.M.; O’Sullivan, B.; Starczynski, J.; Tanchel, B.; Mir, Y.; Lewis, P.; Shaaban, A.M. Inter- and Intra-Observer Agreement of PD-L1 SP142 Scoring in Breast Carcinoma—A Large Multi-Institutional International Study. Cancers 2023, 15, 1511. https://doi.org/10.3390/cancers15051511
- ↑ "Blockade of endogenous B7-H1 suppresses antibacterial protection after primary Listeria monocytogenes infection". Immunology 123 (1): 90–99. January 2008. doi:10.1111/j.1365-2567.2007.02708.x. PMID 17971153.
- ↑ "Constitutive programmed death ligand 1 expression protects gastric G-cells from Helicobacter pylori-induced inflammation". Helicobacter 27 (5): e12917. October 2022. doi:10.1111/hel.12917. PMID 35899973.
- ↑ "The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice". The Journal of Experimental Medicine 198 (1): 63–69. July 2003. doi:10.1084/jem.20022125. PMID 12847137.
- ↑ "Active systemic lupus erythematosus is associated with failure of antigen-presenting cells to express programmed death ligand-1". Rheumatology 47 (9): 1335–1341. September 2008. doi:10.1093/rheumatology/ken256. PMID 18650228.
External links
- CD274+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: Q9NZQ7 (Programmed cell death 1 ligand 1) at the PDBe-KB.
 |
