Chemistry:Fumonisin B4
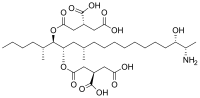 Molecular structure of fumonisin B4
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2R,2′R)-{[(5R,6R,7S,9S,18S,19S)-19-Amino-18-hydroxy-5,9-dimethylicosane-6,7-diyl]bis(oxy)}bis(4-oxobutane-1,2-dicarboxylic acid) | |
| Other names
FB4
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C34H59NO13 | |
| Molar mass | 689.83 g/mol |
| Appearance | White to off-white powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fumonisin B4 (or FB4) is a fumonisin mycotoxin produced mainly by the fungi Fusarium proliferatum, Fusarium verticillioides (formerly Fusarium moniliforme). Recently FB4 has been detected in fungi Aspergillus niger and in several Tolypocladium species.[1]
FB4 is similar to fumonisin B2 and fumonisin B3 but it is lacking a hydroxy group located gamma- to the amino substituent while lacking two hydroxy groups compared to fumonisin B1.[2] Fumonisin B4 has been first published in 1991.[3]
Isomers
Several isomers of Fumonisin B4 have been detected. A larger group is the partially hydrolysed form, denoted as PHFB4, which has been detected using HPLC-ITMS.[4] Another significant isomer is the 3-epi-FB4, which has been identified using NMR, it has also been shown that this isomer occur 10-40% of the regular FB4 sample in nature.[5]
Toxicity
Fumonisin B4 belongs to the class of FB analogues, which is the most significant group from toxicity perspective.[6] Currently Fumonisin B4's toxicity compared to Fumonisin B1 or B2 is unknown as further research is actively ongoing, however in nature its concentration is significantly lower. Fumonisin B4 inhibits sphingosine acyltransferase.
Fumonisin B4 and other fumonisins frequently contaminate maize and other crops.[7]
References
- ↑ Jesper Mølgaard Mogensen; Kirsten Amalie Møller; Pernille Freiesleben; Roman Labuda; Elisabeth Varga; Michael Sulyok; Alena Kuba ́tova; Ulf Thrane et al. (2010). "Production of fumonisins B2 and B4 in Tolypocladium species". Journal of Industrial Microbiology & Biotechnology 38 (9): 1329–35. doi:10.1007/s10295-010-0916-1. PMID 21132348.
- ↑ PubChem. "Fumonisin B4" (in en). PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/42608359.
- ↑ Maria E. Cawood; Wentzel C. A. Gelderblom; Robert Vleggaar; Yosef Behrend; Pieter G. Thiel; Walter F. O. Marasas (1991). "Isolation of the Fumonisin Mycotoxins: A Quantitative Approach". Journal of Agricultural and Food Chemistry 39 (11): 1958–1962. doi:10.1021/jf00011a014.
- ↑ Tibor Bartok; Arpad Szecsi; Andras Szekeres; Akos Mesterhazy; Mihaly Bartok (2006). "Detection of new fumonisin mycotoxins and fumonisin-like compounds by reversed-phase high-performance liquid chromatography/electrospray ionization ion trap mass spectrometry". Rapid Communications in Mass Spectrometry 20 (16): 2447–62. doi:10.1002/rcm.2607. PMID 16871522. Bibcode: 2006RCMS...20.2447B.
- ↑ Wentzel C. A. Gelderblom; Vikash Sewram; Gordon S. Shephard; Petra W. Snijman; Kenny Tenza; Liana van der Westhuizen; Robert Vleggaar (2007). "Structure and Natural Occurrence of Stereoisomers of the Fumonisin B Series Mycotoxins". Journal of Agricultural and Food Chemistry 55 (11): 4388–4394. doi:10.1021/jf070061h. PMID 17469843.
- ↑ John P. Rheeder; Walter F. O. Marasas; Hester F. Vismer (2002). "Production of Fumonisin Analogs by Fusarium Species". Applied and Environmental Microbiology 68 (5): 2101–2105. doi:10.1128/AEM.68.5.2101-2105.2002. PMID 11976077. Bibcode: 2002ApEnM..68.2101R.
- ↑ Jeong-Ah Seo; Yin-Won Lee (1999). "Natural Occurrence of the C Series of Fumonisins in Moldy Corn". Applied and Environmental Microbiology 65 (3): 1331–1334. doi:10.1128/AEM.65.3.1331-1334.1999. PMID 10049903. Bibcode: 1999ApEnM..65.1331S.
 |

