Chemistry:Anisatin
| |
| Names | |
|---|---|
| Preferred IUPAC name
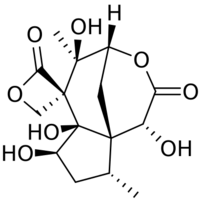
(1R,4S,5R,6S,6aR,7R,9R,9aS)-1,5,6a,7-Tetrahydroxy-5,9-dimethylhexahydrospiro[[4,9a]methanocyclopenta[d]oxocine-6,3′-oxetane]-2,2′(1H)-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | Anisatin |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H20O8 | |
| Molar mass | 328.317 g·mol−1 |
| log P | -1.894 |
| Acidity (pKa) | 12.005 |
| Basicity (pKb) | 1.992 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Anisatin is an extremely toxic, insecticidally active component of the shikimi plant.[1][2] The lethal dose is 1 mg/kg (i.p.) in mice.[3] Symptoms begin to appear about 1–6 hours after ingestion, beginning with gastrointestinal ailments, such as diarrhea, vomiting, and stomach pain, followed by nervous system excitation, seizures, loss of consciousness, and respiratory paralysis, which is the ultimate cause of death.[4]
Role in the GABA system
The GABA system is an important site of action by a variety of chemicals, including alcohols, heavy metals, and insecticides.[5][6] A study conducted on frog spinal cords and rat brains indicated that anisatin was a strong non-competitive GABA antagonist.[5] Anisatin was shown to suppress GABA-induced signals, but when anisatin was added without GABA, there was no change in the signal.[5] Anisatin was also found to share the same binding site as picrotoxinin, and did not cause additional suppression of GABA-induced signals in the presence of high concentrations of picrotoxinin.[5]
Anisatin poisoning has been shown to cause epilepsy, hallucinations, nausea, and convulsions.[7][8] Diazepam has been studied as an anti-convulsive on the GABA system, and has been shown to be an effective treatment for anisatin-induced convulsions.[8]
Synthesis
A total synthesis of (-)-anisatin was reported in 1990.[9]
References
- ↑ "Anisatin". PubChem, National Library of Medicine, US National Institutes of Health. 11 May 2019. https://pubchem.ncbi.nlm.nih.gov/compound/115121.
- ↑ Lane, John F.; Koch, Walter T.; Leeds, Norma S.; Gorin, George (1952). "The toxin of Illicium anisatum. I. The isolation and characterization of a convulsant principle: anisatin". Journal of the American Chemical Society 74 (13): 3211–3215. doi:10.1021/ja01133a002.
- ↑ Kouno, Isao; Kawano, Nobusuke; Yang, Chun-Shu (1988). "New pseudoanisatin-like sesquiterpene lactones from the bark of Illicium dunnianum". Journal of the Chemical Society, Perkin Transactions 1 (6): 1537. doi:10.1039/P19880001537.
- ↑ "Naoru.com:シキミ(Japanese)". http://www.naoru.com/sikimi.htm.
- ↑ 5.0 5.1 5.2 5.3 "Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons". British Journal of Pharmacology 127 (7): 1567–76. August 1999. doi:10.1038/sj.bjp.0702700. PMID 10455311.
- ↑ Buckingham, SD; Ihara, M; Sattelle, DB; Matsuda, K (2017). "Mechanisms of Action, Resistance and Toxicity of Insecticides Targeting GABA Receptors". Current Medicinal Chemistry 24 (27): 2935–45. doi:10.2174/0929867324666170613075736. ISSN 1875-533X. PMID 28606041. https://pubmed.ncbi.nlm.nih.gov/28606041/.
- ↑ "(1)H NMR metabolomics to study the effects of diazepam on anisatin induced convulsive seizures". Journal of Pharmaceutical and Biomedical Analysis 117: 184–94. January 2016. doi:10.1016/j.jpba.2015.08.029. PMID 26361344.
- ↑ 8.0 8.1 "Rapid control of Chinese star anise fruits and teas for neurotoxic anisatin by Direct Analysis in Real Time high resolution mass spectrometry". Journal of Chromatography A 1259: 179–86. October 2012. doi:10.1016/j.chroma.2012.03.058. PMID 22484123.
- ↑ Niwa, Haruki; Nisiwaki, Masanori; Tsukada, Itaru; Ishigaki, Takeshi; Ito, Shigeki; Wakamatsu, Kazumasa; Mori, Tatsuya; Ikagawa, Megumi et al. (November 1990). "Stereocontrolled total synthesis of (-)-anisatin: a neurotoxic sesquiterpenoid possessing a novel spiro .beta.-lactone" (in EN). Journal of the American Chemical Society 112 (24): 9001–9003. doi:10.1021/ja00180a067. ISSN 0002-7863.
External links
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
 |
