Chemistry:Zeranol
 | |
| Clinical data | |
|---|---|
| Trade names | Frideron, Ralabol, Ralgro, Ralone, Zerano |
| Other names | Zearanol; α-Zearalanol; Zearalanol; MK-188; P-1496 |
| Routes of administration | By mouth |
| Drug class | Nonsteroidal estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
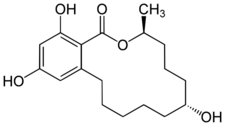
| Formula | C18H26O5 |
| Molar mass | 322.401 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Zeranol (INN, USAN, BAN) (brand names Frideron, Ralabol, Ralgro, Ralone, Zerano; developmental code names MK-188, P-1496), or zearanol, also known as α-zearalanol or simply zearalanol, is a synthetic nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in fungi in the Fusarium genus and is used mainly as an anabolic agent in veterinary medicine.[1][2][3]
Zeranol is approved for use as a growth promoter in livestock, including beef cattle, under the brand name Ralgro (by Merck Animal Health) in the United States .[4] In Canada , it is approved for use in beef cattle only.[5] Its application is not approved for use in the European Union. However, it is marketed under the brand name Ralone in Spain .[2]
Although zeranol may increase cancer cell proliferation in already existing breast cancer,[6] dietary exposure from the use of zeranol-containing implants in cattle is insignificant.[7] Zeranol may be found as a contaminant in fungus-infected crops. It is 3 to 4 times more potent as an estrogen than the related compound zearalenone.[8] It is a metabolite of zearalenone.[9]
See also
- α-Zearalenol
- β-Zearalenol
- Taleranol
- Zearalanone
- Beef hormone controversy
References
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 350–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA350.
- ↑ 2.0 2.1 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1105–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA1105.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 295–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA295.
- ↑ "Implant Strategies for Finishing Cattle using Revalor® (trenbolone acetate and estradiol), Finaplix® (trenbolone) and/or Ralgro® (zeranol)". http://www.depts.ttu.edu/afs/implantdb/dbhome/Revalor%20Tech%20Bulletin%2012.pdf.
- ↑ Health Canada, Questions and Answers - Hormonal Growth Promoters
- ↑ "Mitogenic activity of zeranol in human breast cancer cells is enhanced by leptin and suppressed by gossypol". Anticancer Research 29 (11): 4621–4628. November 2009. PMID 20032412.
- ↑ "Zeranol--a 'nature-identical' oestrogen?". Food and Chemical Toxicology 23 (8): 767–774. August 1985. doi:10.1016/0278-6915(85)90273-x. PMID 2931335.
- ↑ "Incidence of zearalenol (Fusarium mycotoxin) in animal feed". Applied and Environmental Microbiology 38 (4): 749–750. October 1979. doi:10.1128/AEM.38.4.749-750.1979. PMID 161492. Bibcode: 1979ApEnM..38..749M.
- ↑ "Ovine metabolism of zearalenone to α-zearalanol (zeranol).". Journal of Agricultural and Food Chemistry 44 (10): 3244–3250. October 1996. doi:10.1021/jf9601325.
 |
