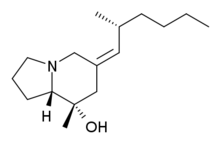
Chemistry:Pumiliotoxin 251D
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6Z,8S,8aS)-8-Methyl-6-[(2R)-2-methylhexylidene]octahydroindolizin-8-ol | |
| Other names
Pumiliotoxin 251D
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C16H29NO | |
| Molar mass | 251.414 g·mol−1 |
| Hazards | |
| Main hazards | Toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pumiliotoxin 251D is a toxic organic compound. It is found in the skin of poison frogs from the genera Dendrobates, Epipedobates, Minyobates, and Phyllobates[1][2] and toads from the genus Melanophryniscus.[3] Its name comes from the pumiliotoxin family (PTXs) and its molecular mass of 251 daltons. When the toxin enters the bloodstream through cuts in the skin or by ingestion,[4] it can cause hyperactivity, convulsions, cardiac arrest and ultimately death. It is especially toxic to arthropods (e.g. mosquitoes), even at low (naturally occurring) concentrations.[5]
Chemical properties
Structure
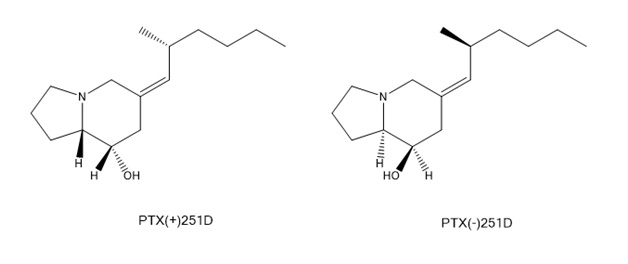
The chiral centers in pumiliotoxin 251D can give several stereoisomers of the compound. Only one form of the toxin is present in nature and has toxic properties.
Two enantiomers of pumiliotoxin 251D. On the left the plus enantiomer is shown which is toxic. On the right side the minus enantiomer, which is not toxic, is shown.
The side chain conformation of substituents at the C-2’ position plays an important role in the toxicity of the compound.[6]
Synthesis
The synthesis of pumiliotoxin 251D is quite complex and contains multiple steps.
One of the starting materials of the synthesis include the N-Boc derivative of L-proline methyl ester (1). Then, a Wittig type of reaction followed by dehydration with thionyl chloride and pyridine results in alkene 2. When alkene 2 undergoes epoxidation with m-chloroperbenzoic acid (MCPBA), epoxide 3 is formed. This then reacts with the lithium salt of dibromoalkene (6) to afford compound 7. Deprotection of compound 7 followed by cyclization and iodination results in vinyl iodide 8. After purification, this yields the hydrochloride of pumiliotoxin (+)-251D (9).
Pumiliotoxin (−)-251D can be synthesized in a similar way with minor alterations to the overall synthesis.[6]
Accumulation
Like many other frog poisons, pumiliotoxin 251D originates from arthropods.[6][7][8] The frogs have a diet of insects that could contain the toxin then it is accumulated in secretory granular glands of the skin of the frog.[6] Some frog species of the Dendrobates genera can convert pumiliotoxin 251D in allopumiliotoxin 267A which is five times more toxic than pumiliotoxin 251D.[6][9] Only one of the enantiomers can be hydroxylated to this more potent form of the toxin.
The lack of pumiliotoxin 251D in eggs and tadpoles confirms that the toxin is not passed over from adult frogs to their offspring.[4] The tadpoles are therefore not readily protected from predators.
Toxicity
Mechanism of action
In general, pumiliotoxins are known as positive modulators of voltage-gated sodium channels (VGSCs, membrane proteins). Pumiliotoxin 251D is not such a poison. However, it does block the influx of Na+ ions in mammalian VGSCs.
Pumiliotoxin 251D is able to shift the V1/2. This is the potential at which the sodium open probability is half maximal. Both the steady-state activation and inactivation curves of each mammalian VGSCs are shifted to a more negative potential.[1]
PTX 251D shifts the V1/2 of insect VGSCs even further than the mammalian VSGCs. This explains why it is especially toxic to insects, like mosquitoes. Furthermore, the presence of PTX 251D results in a six time higher permeability of the VGSCs for K+ ions. This severely disturbs the delicate sodium-potassium equilibrium in the nerve system.
The effect of pumiliotoxin 251D on the voltage-gated potassium channels (VGPCs) currents is quite small. The toxic has an effect on the deactivation kinetics of the potassium channel. It inhibits its inactivation. This effect is still under investigation.[1]
PTX 251D also completely inhibits the activity of Ca2+-stimulated ATPase.[10] This results in a decreased reuptake of Ca2+ and thus a high concentration of free Ca2+ in the organism. This may be related to the potentiation and prolongation of muscle twitch caused by the inhibition.[10]
The mechanism of biotransformation of PTX 251D is still unknown.
Effects
Pumiliotoxin is a toxin found in poison dart frogs (genus Dendrobates and Phyllobates). It affects the calcium channels, interfering with muscle contraction in the heart and skeletal muscle.
PTX 251D has several effects. It rapidly induces convulsions and death to mice and insects (LD50 being, respectively, 10 mg/kg and 150 ng/larvae).[1] These convulsions are the result of the uncontrollable distortion of the sodium-potassium equilibrium in the neurons. This is caused by the inhibition of the VGSCs.
It also acts as cardiac depressor, causing cardiac arrest. This can be explained by its negative effect on the cardiac VGSC hNav1.5/β1.[1]
Although nothing is known of how well PTX 251D penetrates into the brain where convulsions are originated, the observation of convulsions can be explained through inhibition of VGPCs.[1]
Treatment
Symptomatic treatment of PTX 251D poisoning include reducing the convulsions using carbamazepine. This drug targets the affected VGSCs. Phenobarbital also shows positive effects by interacting with the affected Ca2+ channels. Ineffective drugs include diazepam and dizocilpine.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Modulation of voltage-gated Na+ and K+ channels by pumiliotoxin 251D: a "joint venture" alkaloid from arthropods and amphibians". Toxicon 51 (3): 334–44. March 2008. doi:10.1016/j.toxicon.2007.10.011. PMID 18061227. https://zenodo.org/record/1230154.
- ↑ "Main alkaloids from the Brazilian dendrobatidae frog Epipedobates flavopictus: pumiliotoxin 251D, histrionicotoxin and decahydroquinolines". Toxicon 43 (3): 303–10. March 2004. doi:10.1016/j.toxicon.2004.01.001. PMID 15033329.
- ↑ "Further studies on pumiliotoxin 251D and hydroquinone content of the skin secretion of Melanophryniscus species (Anura, Bufonidae) from Uruguay". Toxicon 50 (1): 166–9. July 2007. doi:10.1016/j.toxicon.2007.02.017. PMID 17433395.
- ↑ 4.0 4.1 "Poison Dart Frog – The Deadliest Frog on Earth". FactsList.net. 10 February 2013. http://factslist.net/2013/02/poison-dart-frog-the-deadliest-frog-on-earth/.
- ↑ "A common pumiliotoxin from poison frogs exhibits enantioselective toxicity against mosquitoes". Proceedings of the National Academy of Sciences of the United States of America 103 (47): 17818–21. November 2006. doi:10.1073/pnas.0608646103. PMID 17095598. Bibcode: 2006PNAS..10317818W.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid poison frogs of the genus Dendrobates". Proceedings of the National Academy of Sciences of the United States of America 100 (19): 11092–7. September 2003. doi:10.1073/pnas.1834430100. PMID 12960405. Bibcode: 2003PNAS..10011092D.
- ↑ "Formicine ants: An arthropod source for the pumiliotoxin alkaloids of dendrobatid poison frogs". Proceedings of the National Academy of Sciences of the United States of America 101 (21): 8045–50. May 2004. doi:10.1073/pnas.0402365101. PMID 15128938.
- ↑ "Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs". Journal of Chemical Ecology 31 (10): 2403–15. October 2005. doi:10.1007/s10886-005-7109-9. PMID 16195851.
- ↑ "Toxins". World of Frogs. http://theworldofrogs.weebly.com/toxins.html.
- ↑ 10.0 10.1 "Inhibition of calcium-dependent ATPase from sarcoplasmic reticulum by a new class of indolizidine alkaloids, pumiliotoxins A, B, and 251D". Journal of Neurochemistry 37 (3): 775–80. September 1981. doi:10.1111/j.1471-4159.1982.tb12554.x. PMID 6456330.
 |