Chemistry:JNJ-Q2
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, IV |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
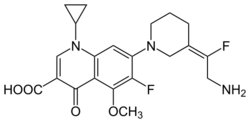
| Formula | C21H23F2N3O4 |
| Molar mass | 419.429 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
JNJ-Q2 is a broad-spectrum fluoroquinolone antibacterial drug being developed for the treatment of acute bacterial skin and skin-structure infections and community-acquired pneumonia. Specifically, JNJ-Q2 is being actively studied for treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections.[2][3]
Furiex Pharmaceuticals has licensed JNJ-Q2 from Janssen Pharmaceutica, a unit of Johnson & Johnson, which discovered JNJ-Q2. Furiex is responsible for its development and commercialization. Both oral and intravenous formulations are being developed.[4]
As of 2016, tests are ongoing.[5]
References
- ↑ "CSID:9721013". ChemSpider. http://www.chemspider.com/Chemical-Structure.9721013.html.
- ↑ "JNJ-Q2, a new fluoroquinolone with potent in vitro activity against Staphylococcus aureus, including methicillin- and fluoroquinolone-resistant strains". Antimicrobial Agents and Chemotherapy 55 (7): 3631–4. July 2011. doi:10.1128/AAC.00162-11. PMID 21555765.
- ↑ "In vitro antibacterial activities of JNJ-Q2, a new broad-spectrum fluoroquinolone". Antimicrobial Agents and Chemotherapy 54 (5): 1955–64. May 2010. doi:10.1128/AAC.01374-09. PMID 20176911.
- ↑ "Novel Fluoroquinolone (JNJ-Q2)". Furiex Pharmaceuticals. http://www.furiex.com/pipeline/discoverydevelopment-pipeline/fluoroquinolone/.
- ↑ "Focus on JNJ-Q2, a novel fluoroquinolone, for the management of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections". Infection and Drug Resistance 9: 119–28. 2016. doi:10.2147/IDR.S105620. PMID 27354817.
