Chemistry:Sodium dihydrogen arsenate
From HandWiki
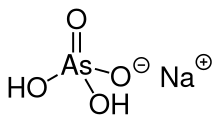
| |
| Names | |
|---|---|
| IUPAC name
Sodium dihydrogen arsorate
| |
| Other names
sodium arsenate monobasic, sodium dihydroarsenate
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| NaH4AsO5 (monohydrate) | |
| Molar mass | 181.9 g/mol |
| Appearance | colourless solid |
| Density | 2.53 g/cm3 |
| slightly soluble | |
| Hazards | |
| Main hazards | toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium dihydrogen arsenate is the inorganic compound with the formula NaH2AsO4. Related salts are also called sodium arsenate, including Na2HAsO4 (disodium hydrogen arsenate) and NaH2AsO4 (sodium dihydrogen arsenate). Sodium dihydrogen arsenate is a colorless solid that is highly toxic.
The salt is the conjugate base of arsenic acid:
- H3AsO4 ⇌ H2AsO−4 + H+ (K1 = 10−2.19)
In the laboratory, it is prepared in this way, crystallizing from a hot saturated aqueous solution, where it is highly soluble when hot (75.3 g in 100 mL at 100 °C). It is obtained as the monohydrate.
Upon heating, solid NaH2AsO4H2O, loses water of crystallization and converts to the pyroarsenate salt Na2H2As2O7.[1]
References
- ↑ Schenk, P. W. (1963). Brauer, G.. ed. Arsenic, Antimony, Bismuth" in Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). New York: Academic Press. p. 602. https://archive.org/details/Handbook_of_Preparative_Inorganic_Chemistry_1_2_Brauer.
 |

