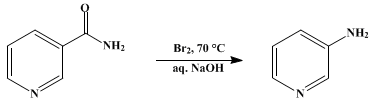Chemistry:Sodium hypobromite
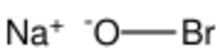
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| NaBrO | |
| Molar mass | 118.893 |
| Appearance | orange solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium hypobromite is the inorganic compound with the formula NaBrO. It is usually obtained as the pentahydrate, so the material that is usually called sodium hypobromite has the formula NaOBr • 5H2O. It is a yellow-orange solid that is soluble in water. It adopts a monoclinic crystal structure with a Br–O bond length of 1.820 Å.[1] It is the Na+ salt of OBr−. It is the bromine analogue of sodium hypochlorite, the active ingredient in common bleach. In practice the salt is usually encountered as an aqueous solution.
Sodium hypobromite arises by treatment of aqueous solution of bromine with base:[2]
- Br2 + 2 NaOH → NaBr + NaBrO + H2O
It can be prepared in situ for use as a reagent, such as in the synthesis of 3-aminopyridine from nicotinamide[3] (Hofmann rearrangement).
References
- ↑ Topić, Filip; Marrett, Joseph M.; Borchers, Tristan H.; Titi, Hatem M.; Barrett, Christopher J.; Friščić, Tomislav (2021). "After 200 Years: The Structure of Bleach and Characterization of Hypohalite Ions by Single-Crystal X-Ray Diffraction". Angew. Chem. Int. Ed. 60 (46): 24400–24405. doi:10.1002/anie.202108843. PMID 34293249.
- ↑ Schmeisser, M. (1963). "Sodium Hypobromite". in Brauer, Georg. Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). New York: Academic Press. pp. 310–311. ISBN 9780323161275. https://books.google.com/books?id=kaa2qeFRXmUC&pg=PA310.
- ↑ Allen, C. F. H.; Wolf, Calvin N. (1950). "3-Aminopyridine". Organic Syntheses 30: 3. doi:10.15227/orgsyn.030.0003. http://www.orgsyn.org/demo.aspx?prep=CV4P0045.; Collective Volume, 4, pp. 45
 |