Chemistry:Sodium metaborate
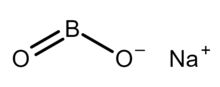 Sodium metaborate monomer
| |
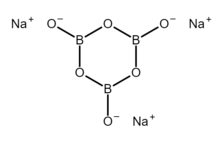 Sodium metaborate trimer
| |
| Names | |
|---|---|
| IUPAC name
Sodium metaborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| NaBO 2 | |
| Molar mass | 65.80 g·mol−1 |
| Appearance | Colorless crystals |
| Odor | Odorless |
| Density | 2.464 g/cm3 (anhydrous)[1] |
| Melting point | 966 °C (1,771 °F; 1,239 K) |
| Boiling point | 1,434[2] °C (2,613 °F; 1,707 K) |
| 16.4 g/(100 mL) (0 °C) 28.2 g/(100 mL) (25 °C) 125.2 g/(100 mL) (100 °C) | |
| Solubility | insoluble in ether, ethanol |
| Structure | |
| trigonal | |
| Thermochemistry | |
Heat capacity (C)
|
65.94 J/(mol·K) |
Std molar
entropy (S |
73.39 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−1059 kJ/mol |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2330 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium metaborate is a chemical compound of sodium, boron, and oxygen with formula NaBO
2.[3] However, the metaborate ion is trimeric in the anhydrous solid, therefore a more correct formula is Na
3B
3O
6 or (Na+
)
3[B
3O
6]3−. The formula can be written also as Na
2O · B
2O
3 to highlight the relation to the main oxides of sodium and boron.[2] The name is also applied to several hydrates whose formulas can be written NaBO
2 · nH
2O for various values of n.
The anhydrous and hydrates are colorless crystalline solids. The anhydrous form is hygroscopic.[4]
Hydrates and solubility
The following hydrates crystallize from solutions of the proper composition in various temperature ranges:[5]
- tetrahydrate NaBO
2 · 4H2O from −6 to 53.6 °C - dihydrate NaBO
2 · 2H2O from 53.6 °C to 105 °C - hemihydrate NaBO
2 · 0.5H2O from 105 °C to the boiling point.
Early reports of a monohydrate NaBO
2 · H2O have not been confirmed.[5]
Structure
Anhydrous
Solid anhydrous sodium metaborate has the hexagonal crystal system with space group . It actually contains a six-membered rings with the formula [B
3O
6]3−, consisting of alternating boron and oxygen atoms with one negatively charged extra oxygen atom attached to each boron atom.[6][1] All nine atoms lie on a plane.[4] The six oxygen atoms are evenly divided into two distinct structural sites, with different B–O bond lengths: B–O(external) 128.0 pm and B–O(bridge) 143.3 pm.[6] The density is 2.348 ± 0.005 g/cm3.[4] The approximate dimensions of the hexagonal cell are a = 1275 pm, c = 733 pm.[4] However, the true unit cell is rhombohedral and has dimensions: ar= 776 pm, α = 110.6°, Z = 6 (5.98) molecules KB0[4]
Dihydrate
The dihydrate NaBO
2 · 2H2O crystallizes in the triclinic crystal system, but is nearly monoclinic, with both α and γ very close to 90°. The cell parameters are a = 678 pm , b = 1058A pm, c = 588 pm, α = 91.5°, β = 22.5°, γ = 89°, Z = 4, density 1.905 g/cm3. The refractive indices at 25°C and wavelength 589.3 nm are α = 1.439, β = 1.473, γ = 1.484. The dispersion is strong, greater at red than at violet.[7]
The transition temperature between the dihydrate and the hemihydrate is 54 °C. However, the crystalline dihydrate will remain metastable until 106 °C to 110 °C, and change slowly above that temperature.[7]
Vapor
Infrared spectroscopy of the vapor from anhydrous sodium metaborate, heated to between 900 °C and 1400 °C, shows mostly isolated clusters with formula NaBO
2, and some dimers thereof.[8] Electron diffraction studies by Akishin and Spiridonov showed a structure O=B–O–Na with linear anion O=B–O−
and angle B–O–Na of 90-110°. The atomic distances are O=B: 120 pm, B–O: 136 pm,O–Na: 214 pm[9]
Preparation
Sodium metaborate is prepared by the fusion of sodium carbonate and boron oxide B
2O
3[1][4] or borax Na
2B
4O
7. Another way to create the compound is by the fusion of borax with sodium hydroxide at 700 °C:
- B
2O
3 + 2 NaOH → 2 NaBO
2 + H
2O
The boiling point of sodium metaborate (1434 °C) is lower than that of boron oxide (1860 °C) and borax (1575 °C) In fact, while the metaborate boils without change of composition, borax gives off a vapor of sodium metaborate with a small excess of sodium oxide Na
2O.[2]
The anhydrous salt can also be prepared from the tetraborate by heating to 270 °C in vacuum.[10]
Although not performed industrially, hydrolysis of sodium borohydride Na[BH
4] with a suitable catalyst gives sodium metaborate and hydrogen gas:[11]
- Na[BH
4] + 2 H
2O → NaBO
2 + 4 H
2 (ΔH = −217 kJ/mol)
Reactions
With water
When sodium metaborate is dissolved in water, the anion combines with two water molecules to form the tetrahydroxyborate anion [B(OH)
4]−
.[11]
Electrochemical conversion to borax
Electrolysis of a concentrated aqueous solution of 20%[clarification needed] NaBO
2 · 4H2O with an anion exchange membrane and inert anode (such as gold, palladium, or boron-doped diamond) converts the metaborate anion to tetraborate B
4O2−
7, and the sodium salt of the later (borax) precipitates as a white powder.[12]
- BO2−
2 + 2 OH−
→ B
4O2−
7 + H
2O + 4 e−
[clarification needed]
Reduction to sodium borohydride
Sodium metaborate can be converted to sodium borohydride by several methods, including the reaction with various reducing agents at high temperatures and pressure,[12] or with magnesium hydride MgH
2 by ball milling at room temperature, followed by extraction of the Na[BH
4] with isopropylamine.[13][10]
- NaBO
2 + 2 MgH
2 → Na[BH
4] + 2 MgO
Another method is the electrolytic reduction of a concentrated sodium metaborate solution,[10] namely
- BO−
2 + 6 H
2O + 8 e−
→ [BH
4]−
+ 8 OH−
However, this method is not efficient since it competes with the reduction of hydroxide:
- 4 OH−
→ 2 H
2O + O
2 + 4 e−
Nanofiltration membranes can effectively separate the borohydride from the metaborate.[11]
Reaction with alcohols
Anhydrous sodium metaborate refluxed with methanol yields the corresponding sodium tetramethoxyborate (melting point: 253-258 °C, CAS number: 18024-69-6[14]):[15]
- Na+
BO−
2 + 4 CH
3OH → Na+
[B(OCH
3)
4]−
+ 2 H
2O
The analogous reaction with ethanol yields the sodium tetraethoxyborate.[15]
Uses
Current and proposed applications of sodium metaborate include:
- Manufacture of borosilicate glasses, which are resistant to uneven or fast heating because of their small coefficient of thermal expansion.
- Composition of herbicides.[16]
- Raising the pH of injected fluids for oil extraction.[17]
See also
References
- ↑ 1.0 1.1 1.2 Ssu-Mien Fang (1938): "The Crystal Structure of Sodium Metaborate Na3(B3O6)". Zeitschrift für Kristallographie - Crystalline Materials, volume 99, issue 1-6, pages 1–8, doi:10.1524/zkri.1938.99.1.1
- ↑ 2.0 2.1 2.2 Sandford S. Cole and Nelson W. Taylor, "The system Na2O-B2O3, IV: Vapor Pressures of Boric Oxide, Sodium Metaborate, and Sodium Diborate between 1150°C and 1400°C". Journal of the American Ceramic Society, volume 18, issue 1‐12, pages 82-85 doi:10.1111/j.1151-2916.1935.tb19358.x
- ↑ "Sodium metaborate" Substance page at the Chemister website. Accessed on 2022-06-28.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 W. H. Zachariasen (1937): "The Crystal Structure of Potassium Metaborate, K3(B3O6)". Journal of Chemical Physics, volume 5, issue 11, page 919. doi:10.1063/1.1749962
- ↑ 5.0 5.1 Nelson P. Nies and Richard W. Hulbert (1967): "Solubility isotherms in the system sodium oxide-boric oxide-water. Revised solubility-temperature curves of boric acid, borax, sodium pentaborate, and sodium metaborate". Journal of Chemical and Engineering Data, volume 12, issue 3, pages 303-313. doi:10.1021/je60034a005
- ↑ 6.0 6.1 M. Marezio, H. A. Plettinger and W. H. Zachariasen (1963): "The bond lengths in the sodium metaborate structure", Acta Crystallographica, volume 16, pages 594-595. doi:10.1107/S0365110X63001596
- ↑ 7.0 7.1 John Krc, Jr. (1951): "Crystallographic Data. 44. Sodium Metaborate Dihydrate". Analytical Chemistry, volume 23, issue 5, page 806. doi:10.1021/ac60053a043
- ↑ Alfred Büchler and Edward P. Marram (1963): "Gaseous Metaborates. II. Infrared Spectraof Alkali Metaborate Vapors". Journal of Chemical Physics, volume 39, page 292. doi:10.1063/1.173424439
- ↑ P. A. Akishin and V. P. Spirtdonov (1962): "Electron Diffraction Study of the Structure Of LiBO2 and NaBO2 Metaborate Molecules in the Vapor State". Zhumal Struktumoi Khimii, volume 3, issue 3, pages 267-269. doi:10.1007/BF01151477
- ↑ 10.0 10.1 10.2 Lingyan Kong, Xinyu Cui, Huazi Jin, Jie Wu, Hao Du, and Tianying Xiong (2009): "Mechanochemical Synthesis of Sodium Borohydride by Recycling Sodium Metaborate". Energy Fuels, volume 23, issue 10, pages 5049-5054. doi:10.1021/ef900619y
- ↑ 11.0 11.1 11.2 Hasan K. Atiyeh and Boyd R. Davis (2007): "Separation of sodium metaborate from sodium borohydride using nanofiltration membranes for hydrogen storage application". International Journal of Hydrogen Energy, volume 32, issue 2, pages 229-236. doi:10.1016/j.ijhydene.2006.06.003
- ↑ 12.0 12.1 Eun Hee Park, Seong Uk Jeong, Un Ho Jung, Sung Hyun Kim, Jaeyoung Lee, Suk Woo Nam, Tae Hoon Lim, Young Jun Park, Yong Ho Yuc (2007): "Recycling of sodium metaborate to borax". International Journal of Hydrogen Energy, volume 32, issue 14, pages 2982-2987. doi:10.1016/j.ijhydene.2007.03.029
- ↑ Z. P. Li, B. H. Liu. K. Arai, N. Morigazaki, S. Suda (2003): "Protide compounds in hydrogen storage systems". Journal of Alloys and Compounds, volumes 356–357, pages 469-474. doi:10.1016/S0925-8388(02)01241-0
- ↑ "Product". https://www.sigmaaldrich.com/GB/en/product/enamine/enah65927943?context=bbe.
- ↑ 15.0 15.1 T. Kemmitt and G. J. Gainsford (2009): "Regeneration of sodium borohydride from sodium metaborate, and isolation of intermediate compounds" International Journal of Hydrogen Energy, volume 34, issue 14, pages 5726-5731. doi:10.1016/j.ijhydene.2009.05.108
- ↑ "BareSpot Monobor-Chlorate". Product safety data sheet at the BareSpot company website.Retrieved 2022-06-28.
- ↑ Fuzhen Chen, Hanqiao Jiang, Xiaohu Bai, Wei Zheng (2013): "Evaluation the performance of sodium metaborate as a novel alkali in alkali/surfactant/polymer flooding". Journal of Industrial and Engineering Chemistry, volume 19, issue 2, 25 March , Pages 450-457. doi:10.1016/j.jiec.2012.08.029
 |
