Chemistry:Sodium perchlorate
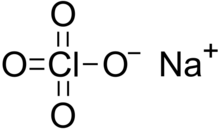
| |
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1502 |
| |
| |
| Properties | |
| NaClO 4 (anhydrous) NaClO 4 · H2O (monohydrate) | |
| Molar mass | 122.44 g/mol (anhydrous) 140.45 g/mol (monohydrate) |
| Appearance | White crystalline solid |
| Density | 2.4994 g/cm3 (anhydrous) 2.02 g/cm3 (monohydrate) |
| Melting point | 468 °C (874 °F; 741 K) (decomposes, anhydrous) 130 °C (monohydrate) |
| Boiling point | 482 °C (900 °F; 755 K) (decomposes, monohydrate) |
| 209.6 g/(100 mL) (25 °C, anhydrous) 209 g/(100 mL) (15 °C, monohydrate) | |
Refractive index (nD)
|
1.4617 |
| Structure | |
| orthorhombic | |
| Hazards | |
| Safety data sheet | ICSC 0715 |
| GHS pictograms |   
|
| GHS Signal word | DANGER |
| H271, H302, H319, H373 | |
| P102, P220, P305+351+338, P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 400 °C (752 °F; 673 K) |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Related compounds
|
Perchloric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium perchlorate is an inorganic compound with the chemical formula NaClO
4. It consists of sodium cations Na+
and perchlorate anions ClO−
4. It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as sodium perchlorate monohydrate NaClO
4 · H2O. The compound is noteworthy as the most water-soluble of the common perchlorate salts.
Sodium perchlorate and other perchlorates has been found on the planet Mars, first detected by the NASA probe Phoenix in 2009. This was later confirmed by spectral analysis by the Mars Reconnaissance Orbiter in 2015 of what is thought to be brine seeps which may be the first evidence of flowing liquid water containing hydrated salts on Mars.[1][2]
Selected properties
Its heat of formation is −382.75 kJ/mol, i.e. it is thermally stable up to high temperatures. At 490 °C it undergoes thermal decomposition, producing sodium chloride and dioxygen.[3] It crystallizes in the rhombic crystal system.[4]
Uses
Perchloric acid is made by treating NaClO
4 with HCl.[5] Ammonium perchlorate and potassium perchlorate, of interest in rocketry and pyrotechnics, are prepared by double decomposition from a solution of sodium perchlorate and ammonium chloride or potassium chloride, respectively.
Laboratory applications
Solutions of NaClO
4 are often used as an unreactive electrolyte. Sodium perchlorate is the precursor to many other perchlorate salts, often taking advantage of their low solubility relative to NaClO
4 (209 g/(100 mL) at 25 °C).[6]
It is used in standard DNA extraction and hybridization reactions in molecular biology.
In medicine
Sodium perchlorate can be used to block iodine uptake before administration of iodinated contrast agents in patients with subclinical hyperthyroidism (suppressed TSH).[7]
Production
Sodium perchlorate is produced by anodic oxidation of sodium chlorate (NaClO
3) at an inert electrode, such as platinum.[5]
- Na+
ClO−
3 + H
2O → Na+
ClO−
4 + 2 H+
+ 2 e−
(acidic medium) - Na+
ClO−
3 + 2 OH−
→ Na+
ClO−
4 + H
2O + 2 e−
(alkaline medium)
Safety
LD50 is 2 – 4 g/kg (rabbits, oral).[5]
See also
References
- ↑ Wadsworth, Jennifer; Cockell, Charles S. (July 6, 2017). "Perchlorates on Mars enhance the bacteriocidal effects of UV light". Scientific Reports 7 (2017, #4662): 4662. doi:10.1038/s41598-017-04910-3. PMID 28684729. Bibcode: 2017NatSR...7.4662W.
- ↑ Delbecq, Denis (September 28, 2015). "De l'eau liquide répérée sur les pentes martiennes" (in fr). https://www.letemps.ch/sciences/2015/09/28/eau-liquide-reperee-pentes-martiennes.
- ↑ Devlin, D. J.; Herley, P. J. (1987). "Thermal decomposition and dehydration of sodium perchlorate monohydrate". Reactivity of Solids 3 (1–2): 75–84. doi:10.1016/0168-7336(87)80019-. https://www.sciencedirect.com/science/article/pii/0168733687800190. Retrieved 3 May 2023.
- ↑ Eagleson, Mary (1994). Concise Encyclopedia Chemistry. revised, illustrated. Walter de Gruyter. p. 1000. ISBN 978-3-11-011451-5. https://archive.org/details/conciseencyclope00eagl. Retrieved March 7, 2013.
- ↑ 5.0 5.1 5.2 Helmut Vogt; Jan Balej; John E. Bennett; Peter Wintzer; Saeed Akbar Sheikh; Patrizio Gallone (2000). "Chlorine Oxides and Chlorine Oxygen Acids". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a06_483. ISBN 3-527-30673-0.
- ↑ Angus, Patricia M.; Jackson, W. Gregory (1994). "Linkage Isomerism in Cobalt(III) Pentaammine Complexes of 2-Pyridone". Inorganic Chemistry 33 (3): 477–483. doi:10.1021/ic00081a014.
- ↑ Becker C. [Prophylaxis and treatment of side effects due to iodinated contrast media relevant to radiological practice]. Radiologe. 2007 Sep;47(9):768-73.
External links
 |


