Chemistry:Zirconium(IV) acetate
| |
| Identifiers | |
|---|---|
| Properties | |
| C24H40O32Zr6 | |
| Molar mass | 1387.896 g·mol−1 |
| Appearance | white solid |
| soluble | |
| Related compounds | |
Related compounds
|
zirconium dioxide, zirconium(IV) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
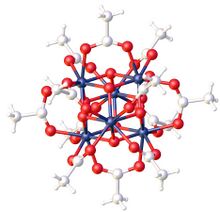
Zirconium acetate usually refers to the chemical formula Zr
6O
4(OH)
4(O
2CCH
3)
12. It forms by the reaction of zirconyl chloride and acetate. Claims of Zr(O
2CCH
3)
4[1] have been superseded by experiments using X-ray crystallography.[2]
The species Zr
6O
4(OH)
4(O
2CCH
3)
12 is a common precursor to metal-organic frameworks (MOFs).[3]
References
- ↑ Pavlov, V. L.; Lysenko, Yu. A.; Kalinichenko, A. A. Synthesis and properties of zirconium tetraacetate.(in Russian) Zhurnal Neorganicheskoi Khimii, 1972. 17(12). ISSN 0044-457X
- ↑ Hennig, Christoph; Weiss, Stephan; Kraus, Werner; Kretzschmar, Jerome; Scheinost, Andreas C. (2017). "Solution Species and Crystal Structure of Zr(IV) Acetate". Inorganic Chemistry 56 (5): 2473–2480. doi:10.1021/acs.inorgchem.6b01624. PMID 28199091.
- ↑ Cavka, Jasmina Hafizovic; Jakobsen, Søren; Olsbye, Unni; Guillou, Nathalie; Lamberti, Carlo; Bordiga, Silvia; Lillerud, Karl Petter (2008). "A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability". Journal of the American Chemical Society 130 (42): 13850–13851. doi:10.1021/ja8057953. PMID 18817383.
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
https://doi.org/10.1021/acs.inorgchem.6b01624 Copyright © 2017 American Chemical Society RIGHTS & PERMISSIONS Subscribed Access Article Views 3592 Altmetric 5 Citations 57 LEARN ABOUT THESE METRICS Share
Add to
Export
RIS PDF (2 MB) OpenURL UNIV ILLINOIS URBANA-CHAMPAIGNSupporting Info (2)»Supporting Information SUBJECTS:Cluster chemistry, Go to Inorganic Chemistry Inorganic Chemistry Abstract
Complex formation and the coordination of zirconium with acetic acid were investigated with Zr K-edge extended X-ray absorption fine structure spectroscopy (EXAFS) and single-crystal diffraction. Zr K-edge EXAFS spectra show that a stepwise increase of acetic acid in aqueous solution with 0.1 M Zr(IV) leads to a structural rearrangement from initial tetranuclear hydrolysis species [Zr4(OH)8(OH2)16]8+ to a hexanuclear acetate species Zr6(O)4(OH)4(CH3COO)12.
 |

