Chemistry:Gilteritinib
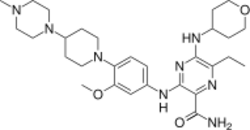 | |
| Clinical data | |
|---|---|
| Trade names | Xospata |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C29H44N8O3 |
| Molar mass | 552.724 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Gilteritinib, sold under the brand name Xospata, is an anti-cancer drug.[6] It acts as an inhibitor of FLT3, hence it is a tyrosine kinase inhibitor.[7]
It was developed by Astellas Pharma.
In April 2018, Astellas filed a new drug application with the Food and Drug Administration for gilteritinib for the treatment of adult patients with FLT3 mutation–positive relapsed or refractory acute myeloid leukemia (AML).[8]
In November 2018, the FDA approved gilteritinib for treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation as detected by an FDA-approved test.[9][4]
Gilteritinib was granted orphan drug status by the U.S. FDA, the European Commission (EC) and the Japan Ministry of Health, Labor and Welfare, for some AML patients.[10]
Gilteritinib was approved for medical use in Australia in March 2020.[11]
References
- ↑ 1.0 1.1 "Xospata Australian prescription medicine decision summary". 11 April 2020. https://www.tga.gov.au/apm-summary/xospata.
- ↑ "Summary Basis of Decision (SBD) for Xospata". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00469&lang=en.
- ↑ "Xospata 40 mg film-coated tablets - Summary of Product Characteristics (SmPC)". 13 November 2019. https://www.medicines.org.uk/emc/product/10832/smpc.
- ↑ 4.0 4.1 "Xospata- gilteritinib tablet". 31 May 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b5ff59aa-9c0d-49a8-9053-1f179b482383.
- ↑ "Xospata EPAR". 16 September 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/xospata.
- ↑ "Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study". The Lancet. Oncology 18 (8): 1061–1075. August 2017. doi:10.1016/S1470-2045(17)30416-3. PMID 28645776.
- ↑ "Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor". Blood 129 (2): 257–260. January 2017. doi:10.1182/blood-2016-10-745133. PMID 27908881.
- ↑ "FDA Approval Sought for Gilteritinib in FLT3+ AML". onclive.com. April 24, 2018. https://www.onclive.com/web-exclusives/fda-approval-sought-for-gilteritinib-in-flt3-aml.
- ↑ "FDA approves gilteritinib for relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation". 2018-11-28. https://www.fda.gov/drugs/fda-approves-gilteritinib-relapsed-or-refractory-acute-myeloid-leukemia-aml-flt3-mutatation.
- ↑ "U.S. FDA Grants Priority Review to Astellas' New Drug Application for Gilteritinib for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia (AML)". https://www.drugs.com/nda/gilteritinib_180529.html.
- ↑ "AusPAR: Gilteritinib (as fumarate)". 11 September 2020. https://www.tga.gov.au/auspar/auspar-gilteritinib-fumarate.
Further reading
- AusPAR: Gilteritinib (as fumarate) (Report). September 2020. https://www.tga.gov.au/auspar/auspar-gilteritinib-fumarate.
External links
- "Gilteritinib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/gilteritinib.
- "Gilteritinib fumarate". NCI Drug Dictionary. National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/792549.
- "Gilteritinib fumarate". National Cancer Institute. 17 January 2019. https://www.cancer.gov/about-cancer/treatment/drugs/gilteritinibfumarate.
 |

