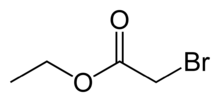
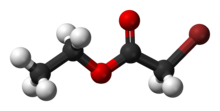
Chemistry:Ethyl bromoacetate
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl bromoacetate | |
| Other names
Ethyl 2-bromoacetate
Bromoacetic acid, ethyl ester Antol Ethoxycarbonylmethyl bromide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1603 |
| |
| |
| Properties | |
| C4H7BrO2 | |
| Molar mass | 167.002 g·mol−1 |
| Appearance | Colorless to yellow liquid[1] |
| Density | 1.51 g/cm3 |
| Melting point | −38 °C (−36 °F; 235 K)[1] |
| Boiling point | 158 °C (316 °F; 431 K)[1] |
| Insoluble | |
| -82.8·10−6 cm3/mol | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H300, H310, H330 | |
| P260, P262, P264, P270, P271, P280, P284, P301+310, P302+350, P304+340, P310, P320, P321, P322, P330, P361, P363, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 47 °C (117 °F; 320 K)[1] |
| Related compounds | |
Other anions
|
Ethyl acetoacetate Ethyl iodoacetate |
Related esters
|
Methyl bromoacetate |
Related compounds
|
Pepper spray Chloropicrin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethyl bromoacetate is the chemical compound with the formula CH2BrCO2C2H5. It is the ethyl ester of bromoacetic acid and is prepared in two steps from acetic acid.[2] It is a lachrymator and has a fruity, pungent odor.[3] It is also a highly toxic alkylating agent and may be fatal if inhaled.
Applications
Ethyl bromoacetate is listed by the World Health Organization as a riot control agent, and was first employed for that purpose by French police in 1912.[4] The French army used rifle grenades 'grenades lacrymogènes'[5] filled with this gas against the Germans beginning in August 1914, but the weapons were largely ineffective, even though ethyl bromoacetate is twice as toxic as chlorine.[6][lower-alpha 1] In the early months of the war the British also used the weaponized use of tear gas agents and more toxic gasses including sulfur dioxide.[7] The German army then used these attacks to justify their subsequent employment of it as odorant or warning agent in odorless, toxic gases and chemical weapons in 1915 under the German code Weisskreuz (White Cross).[8]
In organic synthesis, it is a versatile alkylating agent. Its major application involves the Reformatsky reaction, wherein it reacts with zinc to form a zinc enolate. The resulting BrZnCH2CO2Et condenses with carbonyl compounds to give a β-hydroxy-esters.
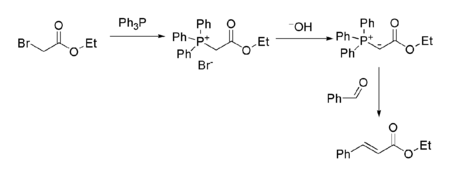
It is also the starting point for the preparation of several other reagents. For example, the related Wittig reagent (prepared by reaction with triphenylphosphine) is commonly used to prepare alpha,beta-unsaturated esters from carbonyl compounds such as benzaldehyde:[9]
References
- ↑ 1.0 1.1 1.2 1.3 Chemicalland properties database (dead link 13 September 2018)
- ↑ Natelson, S.; Gottfried, S. (1955). "Ethyl Bromoacetate". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv3p0381.; Collective Volume, 3, pp. 381
- ↑ Criswell, DW; McClure, FL; Schaefer, R; Brower, KR (1980). "War gases as olfactory probes". Science 210 (4468): 425–6. doi:10.1126/science.6968976. PMID 6968976. Bibcode: 1980Sci...210..425C.
- ↑ Public health response to biological and chemical weapons, Chapter 3, Biological and Chemical agents, WHO Guidance]
- ↑ "Plaidoyer pour la guerre des gaz". 15 April 2005. https://www.nouvelobs.com/culture/20050408.OBS3424/plaidoyer-pour-la-guerre-des-gaz.html.
- ↑ "Plaidoyer pour la guerre des gaz". 15 April 2005. https://www.nouvelobs.com/culture/20050408.OBS3424/plaidoyer-pour-la-guerre-des-gaz.html.
- ↑ "Poison Gas and World War One". http://www.historylearningsite.co.uk/world-war-one/the-western-front-in-world-war-one/poison-gas-and-world-war-one/.
- ↑ Heller, Charles E. (September 1984). "Chemical Warfare in World War I: The American Experience, 1917-1918". Combat Studies Institute. http://www-cgsc.army.mil/carl/resources/csi/Heller/HELLER.asp.
- ↑ A student lab procedure for the Wittig sequence shown, only using the related methyl ester.
Footnotes
- ↑ The small quantities of gas delivered, roughly 19 cm3 per cartridge, were not even detected by the Germans. The stocks were rapidly consumed.[Why bromoacetate failed in WW1]
External links
 |