Medicine:Rheumatoid arthritis
| Rheumatoid arthritis | |
|---|---|
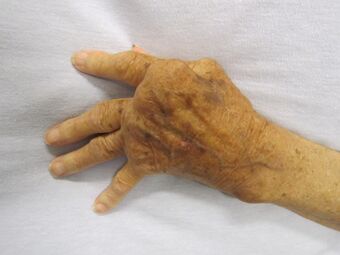 | |
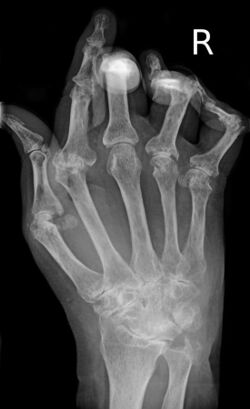
| A hand severely affected by rheumatoid arthritis. This degree of swelling and deformation does not typically occur with current treatment. | |
| Specialty | Rheumatology, Immunology |
| Symptoms | Warm, swollen, painful joints[1] |
| Complications | Low red blood cells, inflammation around the lungs, inflammation around the heart[1] |
| Usual onset | Middle age[1] |
| Duration | Lifelong[1] |
| Causes | Unknown[1] |
| Diagnostic method | Based on symptoms, medical imaging, blood tests[1][2] |
| Differential diagnosis | Systemic lupus erythematosus, psoriatic arthritis, fibromyalgia[2] |
| Medication | Pain medications, steroids, Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs[1] |
| Frequency | 0.5–1% (adults in developed world)[3] |
| Deaths | 30,000 (2015)[4] |
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints.[1] It typically results in warm, swollen, and painful joints.[1] Pain and stiffness often worsen following rest.[1] Most commonly, the wrist and hands are involved, with the same joints typically involved on both sides of the body.[1] The disease may also affect other parts of the body, including skin, eyes, lungs, heart, nerves, and blood.[1] This may result in a low red blood cell count, inflammation around the lungs, and inflammation around the heart.[1] Fever and low energy may also be present.[1] Often, symptoms come on gradually over weeks to months.[2]
While the cause of rheumatoid arthritis is not clear, it is believed to involve a combination of genetic and environmental factors.[1] The underlying mechanism involves the body's immune system attacking the joints.[1] This results in inflammation and thickening of the joint capsule.[1] It also affects the underlying bone and cartilage.[1] The diagnosis is made mostly on the basis of a person's signs and symptoms.[2] X-rays and laboratory testing may support a diagnosis or exclude other diseases with similar symptoms.[1] Other diseases that may present similarly include systemic lupus erythematosus, psoriatic arthritis, and fibromyalgia among others.[2]
The goals of treatment are to reduce pain, decrease inflammation, and improve a person's overall functioning.[5] This may be helped by balancing rest and exercise, the use of splints and braces, or the use of assistive devices.[1][6][7] Pain medications, steroids, and NSAIDs are frequently used to help with symptoms.[1] Disease-modifying antirheumatic drugs (DMARDs), such as hydroxychloroquine and methotrexate, may be used to try to slow the progression of disease.[1] Biological DMARDs may be used when the disease does not respond to other treatments.[8] However, they may have a greater rate of adverse effects.[9] Surgery to repair, replace, or fuse joints may help in certain situations.[1]
RA affects about 24.5 million people as of 2015.[10] This is 0.5–1% of adults in the developed world with between 5 and 50 per 100,000 people newly developing the condition each year.[3] Onset is most frequent during middle age and women are affected 2.5 times as frequently as men.[1] It resulted in 38,000 deaths in 2013, up from 28,000 deaths in 1990.[11] The first recognized description of RA was made in 1800 by Dr. Augustin Jacob Landré-Beauvais (1772–1840) of Paris.[12] The term rheumatoid arthritis is based on the Greek for watery and inflamed joints.[13]
Signs and symptoms
RA primarily affects joints, but it also affects other organs in more than 15–25% of cases.[14] Associated problems include cardiovascular disease, osteoporosis, interstitial lung disease, infection, cancer, feeling tired, depression, mental difficulties, and trouble working.[15]
Joints
Arthritis of joints involves inflammation of the synovial membrane. Joints become swollen, tender and warm, and stiffness limits their movement. With time, multiple joints are affected (polyarthritis). Most commonly involved are the small joints of the hands, feet and cervical spine, but larger joints like the shoulder and knee can also be involved.[16]:1098 Synovitis can lead to tethering of tissue with loss of movement and erosion of the joint surface causing deformity and loss of function.[2] The fibroblast-like synoviocytes (FLS), highly specialized mesenchymal cells found in the synovial membrane, have an active and prominent role in these pathogenic processes of the rheumatic joints.[17]
RA typically manifests with signs of inflammation, with the affected joints being swollen, warm, painful and stiff, particularly early in the morning on waking or following prolonged inactivity. Increased stiffness early in the morning is often a prominent feature of the disease and typically lasts for more than an hour. Gentle movements may relieve symptoms in early stages of the disease. These signs help distinguish rheumatoid from non-inflammatory problems of the joints, such as osteoarthritis. In arthritis of non-inflammatory causes, signs of inflammation and early morning stiffness are less prominent.[18] The pain associated with RA is induced at the site of inflammation and classified as nociceptive as opposed to neuropathic.[19] The joints are often affected in a fairly symmetrical fashion, although this is not specific, and the initial presentation may be asymmetrical.[16]:1098
As the pathology progresses the inflammatory activity leads to tendon tethering and erosion and destruction of the joint surface, which impairs range of movement and leads to deformity. The fingers may develop almost any deformity depending on which joints are most involved. Specific deformities, which also occur in osteoarthritis, include ulnar deviation, boutonniere deformity (also "buttonhole deformity", flexion of proximal interphalangeal joint and extension of distal interphalangeal joint of the hand), swan neck deformity (hyperextension at proximal interphalangeal joint and flexion at distal interphalangeal joint) and "Z-thumb." "Z-thumb" or "Z-deformity" consists of hyperextension of the interphalangeal joint, fixed flexion and subluxation of the metacarpophalangeal joint and gives a "Z" appearance to the thumb.[16]:1098 The hammer toe deformity may be seen. In the worst case, joints are known as arthritis mutilans due to the mutilating nature of the deformities.[20]
Skin
The rheumatoid nodule, which is sometimes in the skin, is the most common non-joint feature and occurs in 30% of people who have RA.[21] It is a type of inflammatory reaction known to pathologists as a "necrotizing granuloma". The initial pathologic process in nodule formation is unknown but may be essentially the same as the synovitis, since similar structural features occur in both. The nodule has a central area of fibrinoid necrosis that may be fissured and which corresponds to the fibrin-rich necrotic material found in and around an affected synovial space. Surrounding the necrosis is a layer of palisading macrophages and fibroblasts, corresponding to the intimal layer in synovium and a cuff of connective tissue containing clusters of lymphocytes and plasma cells, corresponding to the subintimal zone in synovitis. The typical rheumatoid nodule may be a few millimetres to a few centimetres in diameter and is usually found over bony prominences, such as the elbow, the heel, the knuckles, or other areas that sustain repeated mechanical stress. Nodules are associated with a positive RF (rheumatoid factor) titer, ACPA, and severe erosive arthritis. Rarely, these can occur in internal organs or at diverse sites on the body.[22]
Several forms of vasculitis occur in RA, but are mostly seen with long-standing and untreated disease. The most common presentation is due to involvement of small- and medium-sized vessels. Rheumatoid vasculitis can thus commonly present with skin ulceration and vasculitic nerve infarction known as mononeuritis multiplex.[23]
Other, rather rare, skin associated symptoms include pyoderma gangrenosum, Sweet's syndrome, drug reactions, erythema nodosum, lobe panniculitis, atrophy of finger skin, palmar erythema, and skin fragility (often worsened by corticosteroid use).[citation needed]
Diffuse alopecia areata (Diffuse AA) occurs more commonly in people with rheumatoid arthritis.[24] RA is also seen more often in those with relatives who have AA.[24]
Lungs
Lung fibrosis is a recognized complication of rheumatoid arthritis. It is also a rare but well-recognized consequence of therapy (for example with methotrexate and leflunomide). Caplan's syndrome describes lung nodules in individuals with RA and additional exposure to coal dust. Exudative pleural effusions are also associated with RA.[25][26]
Heart and blood vessels
People with RA are more prone to atherosclerosis, and risk of myocardial infarction (heart attack) and stroke is markedly increased.[27][28][29] Other possible complications that may arise include: pericarditis, endocarditis, left ventricular failure, valvulitis and fibrosis.[30] Many people with RA do not experience the same chest pain that others feel when they have angina or myocardial infarction. To reduce cardiovascular risk, it is crucial to maintain optimal control of the inflammation caused by RA (which may be involved in causing the cardiovascular risk), and to use exercise and medications appropriately to reduce other cardiovascular risk factors such as blood lipids and blood pressure. Doctors who treat people with RA should be sensitive to cardiovascular risk when prescribing anti-inflammatory medications, and may want to consider prescribing routine use of low doses of aspirin if the gastrointestinal effects are tolerable.[30]
Blood
Anemia is by far the most common abnormality of the blood cells which can be caused by a variety of mechanisms. The chronic inflammation caused by RA leads to raised hepcidin levels, leading to anemia of chronic disease where iron is poorly absorbed and also sequestered into macrophages. The red cells are of normal size and color (normocytic and Normochromic).[31]
A low white blood cell count usually only occurs in people with Felty's syndrome with an enlarged liver and spleen. The mechanism of neutropenia is complex. An increased platelet count occurs when inflammation is uncontrolled.[32]
Other
The role of the circadian clock in rheumatoid arthritis suggests a correlation between an early morning rise in circulating levels of pro-inflammatory cytokines, such as interleukin-6 and painful morning joint stiffness.[33]
Kidneys
Renal amyloidosis can occur as a consequence of untreated chronic inflammation.[34] Treatment with penicillamine or gold salts such as sodium aurothiomalate are recognized causes of membranous nephropathy.[35]
Eyes
The eye can be directly affected in the form of episcleritis[36] or scleritis, which when severe can very rarely progress to perforating scleromalacia. Rather more common is the indirect effect of keratoconjunctivitis sicca, which is a dryness of eyes and mouth caused by lymphocyte infiltration of lacrimal and salivary glands. When severe, dryness of the cornea can lead to keratitis and loss of vision as well as being painful. Preventive treatment of severe dryness with measures such as nasolacrimal duct blockage is important.[37]
Liver
Liver problems in people with rheumatoid arthritis may be due to the underlying disease process or as a result of the medications used to treat the disease.[38] A coexisting autoimmune liver disease, such as primary biliary cirrhosis or autoimmune hepatitis may also cause problems.[38]
Neurological
Peripheral neuropathy and mononeuritis multiplex may occur. The most common problem is carpal tunnel syndrome caused by compression of the median nerve by swelling around the wrist. Rheumatoid disease of the spine can lead to myelopathy. Atlanto-axial subluxation can occur, owing to erosion of the odontoid process and/or transverse ligaments in the cervical spine's connection to the skull. Such an erosion (>3mm) can give rise to vertebrae slipping over one another and compressing the spinal cord. Clumsiness is initially experienced, but without due care, this can progress to quadriplegia or even death.[39]
Constitutional symptoms
Constitutional symptoms including fatigue, low grade fever, malaise, morning stiffness, loss of appetite and loss of weight are common systemic manifestations seen in people with active RA.
Bones
Local osteoporosis occurs in RA around inflamed joints. It is postulated to be partially caused by inflammatory cytokines. More general osteoporosis is probably contributed to by immobility, systemic cytokine effects, local cytokine release in bone marrow and corticosteroid therapy.[40][41]
Cancer
The incidence of lymphoma is increased, although it is uncommon and associated with the chronic inflammation, not the treatment of RA.[42][43] The risk of non-melanoma skin cancer is increased in people with RA compared to the general population, an association possibly due to the use of immunosuppression agents for treating RA.[44]
Teeth
Periodontitis and tooth loss are common in people with rheumatoid arthritis.[45]
Risk factors
RA is a systemic (whole body) autoimmune disease. Some genetic and environmental factors affect the risk for RA.
Genetic
Worldwide, RA affects approximately 1% of the adult population and occurs one in 1,000 children. Studies show RA primarily affects individuals between the ages of 40–60 years and is seen more commonly in females.[46][47] A family history of RA increases the risk around three to five times; as of 2016, it was estimated that genetics may account for 40–65% of cases of seropositive RA, but only around 20% for seronegative RA.[3] RA is strongly associated with genes of the inherited tissue type major histocompatibility complex (MHC) antigen. HLA-DR4 is the major genetic factor implicated – the relative importance varies across ethnic groups.[48]
Genome-wide association studies examining single-nucleotide polymorphisms have found around one hundred alleles associated with RA risk.[49] Risk alleles within the HLA (particularly HLA-DRB1) genes harbor more risk than other loci.[50] The HLA encodes proteins that control recognition of self- versus non-self molecules. Other risk loci include genes affecting co-stimulatory immune pathways—for example CD28 and CD40, cytokine signaling, lymphocyte receptor activation threshold (e.g., PTPN22), and innate immune activation—appear to have less influence than HLA mutations.[3][51]
Environmental
There are established epigenetic and environmental risk factors for RA.[52][3] Smoking is an established risk factor for RA in Caucasian populations, increasing the risk three times compared to non-smokers, particularly in men, heavy smokers, and those who are rheumatoid factor positive.[53] Modest alcohol consumption may be protective.[54]
Silica exposure has been linked to RA.[55]
Negative findings
No infectious agent has been consistently linked with RA and there is no evidence of disease clustering to indicate its infectious cause,[48] but periodontal disease has been consistently associated with RA.[3]
The many negative findings suggest that either the trigger varies, or that it might, in fact, be a chance event inherent with the immune response.[56]
Pathophysiology
RA primarily starts as a state of persistent cellular activation leading to autoimmunity and immune complexes in joints and other organs where it manifests.[57]
The clinical manifestations of disease are primarily inflammation of the synovial membrane and joint damage, and the fibroblast-like synoviocytes play a key role in these pathogenic processes.[17] Three phases of progression of RA are an initiation phase (due to non-specific inflammation), an amplification phase (due to T cell activation), and chronic inflammatory phase, with tissue injury resulting from the cytokines, IL–1, TNF-alpha, and IL–6.[20]
Non-specific inflammation
Factors allowing an abnormal immune response, once initiated, become permanent and chronic. These factors are genetic disorders which change regulation of the adaptive immune response.[3] Genetic factors interact with environmental risk factors for RA, with cigarette smoking as the most clearly defined risk factor.[53][58]
Other environmental and hormonal factors may explain higher risks for women, including onset after childbirth and hormonal medications. A possibility for increased susceptibility is that negative feedback mechanisms – which normally maintain tolerance – are overtaken by positive feedback mechanisms for certain antigens, such as IgG Fc bound by rheumatoid factor and citrullinated fibrinogen bound by antibodies to citrullinated peptides (ACPA – Anti–citrullinated protein antibody). A debate on the relative roles of B-cell produced immune complexes and T cell products in inflammation in RA has continued for 30 years, but neither cell is necessary at the site of inflammation, only autoantibodies to IgGFc, known as rheumatoid factors and ACPA, with ACPA having an 80% specificity for diagnosing RA.[59] As with other autoimmune diseases, people with RA have abnormally glycosylated antibodies, which are believed to promote joint inflammation.[60][page needed]
Amplification in the synovium
Once the generalized abnormal immune response has become established – which may take several years before any symptoms occur – plasma cells derived from B lymphocytes produce rheumatoid factors and ACPA of the IgG and IgM classes in large quantities. These activate macrophages through Fc receptor and complement binding, which is part of the intense inflammation in RA.[61] Binding of an autoreactive antibody to the Fc receptors is mediated through the antibody's N-glycans, which are altered to promote inflammation in people with RA.[60][page needed]
This contributes to local inflammation in a joint, specifically the synovium with edema, vasodilation and entry of activated T-cells, mainly CD4 in microscopically nodular aggregates and CD8 in microscopically diffuse infiltrates.[62]
Synovial macrophages and dendritic cells function as antigen-presenting cells by expressing MHC class II molecules, which establishes the immune reaction in the tissue.[62]
Chronic inflammation
The disease progresses by forming granulation tissue at the edges of the synovial lining, pannus with extensive angiogenesis and enzymes causing tissue damage.[63] The fibroblast-like synoviocytes have a prominent role in these pathogenic processes.[17] The synovium thickens, cartilage and underlying bone disintegrate, and the joint deteriorates, with raised calprotectin levels serving as a biomarker of these events.[64] Importantly inflammatory events are not limited to synovium but it appear to be systemic, evidence suggest that alterations in T helper profile favoring inflammation such as inflammatory IL-17A producing T helper cells and pathogenic Th17 cells are come from both memory and effector compartment in RA patients peripheral blood.[65]
Cytokines and chemokines attract and accumulate immune cells, i.e. activated T- and B cells, monocytes and macrophages from activated fibroblast-like synoviocytes, in the joint space. By signalling through RANKL and RANK, they eventually trigger osteoclast production, which degrades bone tissue.[3][66][page needed] The fibroblast-like synoviocytes that are present in the synovium during rheumatoid arthritis display altered phenotype compared to the cells present in normal tissues. The aggressive phenotype of fibroblast-like synoviocytes in rheumatoid arthritis and the effect these cells have on the microenvironment of the joint can be summarized into hallmarks that distinguish them from healthy fibroblast-like synoviocytes. These hallmark features of fibroblast-like synoviocytes in rheumatoid arthritis are divided into seven cell-intrinsic hallmarks and four cell-extrinsic hallmarks.[17] The cell-intrinsic hallmarks are: reduced apoptosis, impaired contact inhibition, increased migratory invasive potential, changed epigenetic landscape, temporal and spatial heterogeneity, genomic instability and mutations, and reprogrammed cellular metabolism. The cell-extrinsic hallmarks of FLS in RA are: promotes osteoclastogenesis and bone erosion, contributes to cartilage degradation, induces synovial angiogenesis, and recruits and stimulates immune cells.[17]
Diagnosis
Imaging
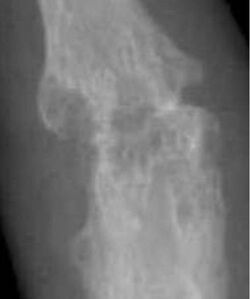
X-rays of the hands and feet are generally performed when many joints affected. In RA, there may be no changes in the early stages of the disease or the x-ray may show osteopenia near the joint, soft tissue swelling, and a smaller than normal joint space. As the disease advances, there may be bony erosions and subluxation. Other medical imaging techniques such as magnetic resonance imaging (MRI) and ultrasound are also used in RA.[20][68]
Technical advances in ultrasonography like high-frequency transducers (10 MHz or higher) have improved the spatial resolution of ultrasound images depicting 20% more erosions than conventional radiography. Color Doppler and power Doppler ultrasound are useful in assessing the degree of synovial inflammation as they can show vascular signals of active synovitis. This is important, since in the early stages of RA, the synovium is primarily affected, and synovitis seems to be the best predictive marker of future joint damage.[69]
Blood tests
When RA is clinically suspected, a physician may test for rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs measured as anti-CCP antibodies).[70][page needed] The test is positive approximately two-thirds of the time, but a negative RF or CCP antibody does not rule out RA; rather, the arthritis is called seronegative, which occurs in approximately a third of people with RA.[71] During the first year of illness, rheumatoid factor is more likely to be negative with some individuals becoming seropositive over time. RF is a non-specific antibody and seen in about 10% of healthy people, in many other chronic infections like hepatitis C, and chronic autoimmune diseases such as Sjögren's syndrome and systemic lupus erythematosus. Therefore, the test is not specific for RA.[20]
Hence, new serological tests check for anti-citrullinated protein antibodies ACPAs. These tests are again positive in 61–75% of all RA cases, but with a specificity of around 95%.[72] As with RF, ACPAs are many times present before symptoms have started.[20]
The by far most common clinical test for ACPAs is the anti-cyclic citrullinated peptide (anti CCP) ELISA. In 2008 a serological point-of-care test for the early detection of RA combined the detection of RF and anti-MCV with a sensitivity of 72% and specificity of 99.7%.[73][better source needed][74]
To improve the diagnostic capture rate in the early detection of patients with RA and to risk stratify these individuals, the rheumatology field continues to seek complementary markers to both RF and anti-CCP. 14-3-3η (YWHAH) is one such marker that complements RF and anti-CCP, along with other serological measures like C-reactive protein. In a systematic review, 14-3-3η has been described as a welcome addition to the rheumatology field. The authors indicate that the serum based 14-3-η marker is additive to the armamentarium of existing tools available to clinicians, and that there is adequate clinical evidence to support its clinical benefits.[75]
Other blood tests are usually done to differentiate from other causes of arthritis, like the erythrocyte sedimentation rate (ESR), C-reactive protein, full blood count, kidney function, liver enzymes and other immunological tests (e.g., antinuclear antibody/ANA) are all performed at this stage. Elevated ferritin levels can reveal hemochromatosis, a mimic of RA, or be a sign of Still's disease, a seronegative, usually juvenile, variant of rheumatoid Arthritis.[76]
Classification criteria
In 2010, the 2010 ACR / EULAR Rheumatoid Arthritis Classification Criteria were introduced.[77]
The new criteria are not diagnostic criteria, but are classification criteria to identify disease with a high likelihood of developing a chronic form.[20] However a score of 6 or greater unequivocally classifies a person with a diagnosis of rheumatoid arthritis.[citation needed]
These new classification criteria overruled the "old" ACR criteria of 1987 and are adapted for early RA diagnosis. The "new" classification criteria, jointly published by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) establish a point value between 0 and 10. Four areas are covered in the diagnosis:[77]
- joint involvement, designating the metacarpophalangeal joints, proximal interphalangeal joints, the interphalangeal joint of the thumb, second through fifth metatarsophalangeal joint and wrist as small joints, and shoulders, elbows, hip joints, knees, and ankles as large joints:
- Involvement of 1 large joint gives 0 points
- Involvement of 2–10 large joints gives 1 point
- Involvement of 1–3 small joints (with or without involvement of large joints) gives 2 points
- Involvement of 4–10 small joints (with or without involvement of large joints) gives 3 points
- Involvement of more than 10 joints (with involvement of at least 1 small joint) gives 5 points
- serological parameters – including the rheumatoid factor as well as ACPA – "ACPA" stands for "anti-citrullinated protein antibody":
- Negative RF and negative ACPA gives 0 points
- Low-positive RF or low-positive ACPA gives 2 points
- High-positive RF or high-positive ACPA gives 3 points
- acute phase reactants: 1 point for elevated erythrocyte sedimentation rate, ESR, or elevated CRP value (c-reactive protein)
- duration of arthritis: 1 point for symptoms lasting six weeks or longer
The new criteria accommodate to the growing understanding of RA and the improvements in diagnosing RA and disease treatment. In the "new" criteria, serology and autoimmune diagnostics carries major weight, as ACPA detection is appropriate to diagnose the disease in an early state, before joints destructions occur. Destruction of the joints viewed in radiological images was a significant point of the ACR criteria from 1987.[78] This criterion no longer is regarded to be relevant, as this is just the type of damage that treatment is meant to avoid.
Differential diagnoses
Several other medical conditions can resemble RA, and need to be distinguished from it at the time of diagnosis:[79]
- Crystal induced arthritis (gout, and pseudogout) – usually involves particular joints (knee, MTP1, heels) and can be distinguished with an aspiration of joint fluid if in doubt. Redness, asymmetric distribution of affected joints, pain occurs at night and the starting pain is less than an hour with gout.
- Osteoarthritis – distinguished with X-rays of the affected joints and blood tests, older age, starting pain less than an hour, asymmetric distribution of affected joints and pain worsens when using joint for longer periods.
- Systemic lupus erythematosus (SLE) – distinguished by specific clinical symptoms and blood tests (antibodies against double-stranded DNA)
- One of the several types of psoriatic arthritis resembles RA – nail changes and skin symptoms distinguish between them
- Lyme disease causes erosive arthritis and may closely resemble RA – it may be distinguished by blood test in endemic areas
- Reactive arthritis – asymmetrically involves heel, sacroiliac joints and large joints of the leg. It is usually associated with urethritis, conjunctivitis, iritis, painless buccal ulcers, and keratoderma blennorrhagica.
- Axial spondyloarthritis (including ankylosing spondylitis) – this involves the spine, although an RA-like symmetrical small-joint polyarthritis may occur in the context of this condition.
- Hepatitis C – RA-like symmetrical small-joint polyarthritis may occur in the context of this condition. Hepatitis C may also induce rheumatoid factor auto-antibodies.
Rarer causes which usually behave differently but may cause joint pains:[79]
- Sarcoidosis, amyloidosis, and Whipple's disease can also resemble RA.
- Hemochromatosis may cause hand joint arthritis.
- Acute rheumatic fever can be differentiated by a migratory pattern of joint involvement and evidence of antecedent streptococcal infection.
- Bacterial arthritis (such as by Streptococcus) is usually asymmetric, while RA usually involves both sides of the body symmetrically.
- Gonococcal arthritis (a bacterial arthritis) is also initially migratory and can involve tendons around the wrists and ankles.
Sometimes arthritis is in an undifferentiated stage (i.e. none of the above criteria is positive), even if synovitis is witnessed and assessed with ultrasound imaging.
Difficult-to-treat
Rheumatoid arthritis (D2T RA) is a specific classification RA by the European League against Rheumatism (EULAR).[80]
Signs of illness:
- Persistence of signs and symptoms
- Drug resistance
- Does not respond on two or more biological treatments
- Does not respond on anti-rheumatic drugs with different mechanism of action
Factors contributing to difficult-to-treat disease:
- Genetic risk factors
- Environmental factors (diet, smoking, physical activity)
- Overweight and obese
Genetic factors
Genetic factors such as HLA-DR1B1,[81] TRAF1, PSORS1C1 and microRNA 146a[82] are associated with difficult to treat rheumatoid arthritis, other gene polymorphisms seem to be correlated with response to biologic modifying anti-rheumatic drugs (bDMARDs). Next one is FOXO3A gene region been reported as associated with worst disorder. The minor allele at FOXO3A summon a differential response of monocytes in RA patients. FOXO3A can provide an increase of pro-inflammatory cytokines, including TNFα. Possible gene polymorphism: STAT4, PTPN2, PSORS1C1 and TRAF3IP2 genes had been correlated with response to TNF inhibitors.[83]
HLA-DR1 and HLA-DRB1 gene
The HLA-DRB1 gene is part of a family of genes called the human leukocyte antigen (HLA) complex. The HLA complex is the human version of the major histocompatibility complex (MHC). Currently, have been identified at least 2479 different versions of the HLA-DRB1 gene.[84] The presence of HLA-DRB1 alleles seems to predict radiographic damage, which may be partially mediated by ACPA development, and also elevated sera inflammatory levels and high swollen joint count. HLA-DR1 is encoded by the most risk allele HLA-DRB1 which share a conserved 5-aminoacid sequence that is correlated with the development of anti-citrullinated protein antibodies.[85] HLA-DRB1 gene have more strong correlation with disease development. Susceptibility to and outcome for rheumatoid arthritis (RA) may associate with particular HLA-DR alleles, but these alleles vary among ethnic groups and geographic areas.[86]
MicroRNAs
MicroRNAs are a factor in the development of that type of disease. MicroRNAs usually operate as a negative regulator of the expression of target proteins and their increased concentration after biologic treatment (bDMARDs) or after anti-rheumatic drugs. Level of miRNA before and after anti-TNFa/DMRADs combination therapy are potential novel biomarkers for predicting and monitoring outcome. For instance, some of them were found significantly upregulated by anti-TNFa/DMRADs combination therapy. For example, miRNA-16-5p, miRNA-23-3p, miRNA125b-5p, miRNA-126-3p, miRNA-146a-5p, miRNA-223-3p. Curious fact is that only responder patients showed an increase in those miRNAs after therapy, and paralleled the reduction of TNFα, interleukin (IL)-6, IL-17, rheumatoid factor (RF), and C-reactive protein (CRP).[87]
Monitoring progression
Many tools can be used to monitor remission in rheumatoid arthritis.
- DAS28: Disease Activity Score of 28 joints (DAS28) is widely used as an indicator of RA disease activity and response to treatment. Joints included are (bilaterally): proximal interphalangeal joints (10 joints), metacarpophalangeal joints (10), wrists (2), elbows (2), shoulders (2) and knees (2). When looking at these joints, both the number of joints with tenderness upon touching (TEN28) and swelling (SW28) are counted. The erythrocyte sedimentation rate (ESR) is measured and the affected person makes a subjective assessment (SA) of disease activity during the preceding 7 days on a scale between 0 and 100, where 0 is "no activity" and 100 is "highest activity possible". With these parameters, DAS28 is calculated as:[88]
[math]\displaystyle{ DAS28=0.56 \times \sqrt{TEN28} + 0.28 \times \sqrt{SW28} + 0.70 \times \ln(ESR) + 0.014 \times SA }[/math]
From this, the disease activity of the affected person can be classified as follows:[88]
| Current DAS28 |
DAS28 decrease from initial value | |||
|---|---|---|---|---|
| > 1.2 | > 0.6 but ≤ 1.2 | ≤ 0.6 | ||
| ≤ 3.2 | Inactive | Good improvement | Moderate improvement | No improvement |
| > 3.2 but ≤ 5.1 | Moderate | Moderate improvement | Moderate improvement | No improvement |
| > 5.1 | Very active | Moderate improvement | No improvement | No improvement |
It is not always a reliable indicator of treatment effect.[89] One major limitation is that low-grade synovitis may be missed.[90]
- Other: Other tools to monitor remission in rheumatoid arthritis are: ACR-EULAR Provisional Definition of Remission of Rheumatoid arthritis, Simplified Disease Activity Index and Clinical Disease Activity Index.[91] Some scores do not require input from a healthcare professional and allow self-monitoring by the person, like HAQ-DI.[92][page needed]
Management
There is no cure for RA, but treatments can improve symptoms and slow the progress of the disease. Disease-modifying treatment has the best results when it is started early and aggressively.[93][47] The results of a recent systematic review found that combination therapy with tumor necrosis factor (TNF) and non-TNF biologics plus methotrexate (MTX) resulted in improved disease control, Disease Activity Score (DAS)-defined remission, and functional capacity compared with a single treatment of either methotrexate or a biologic alone.[94]
The goals of treatment are to minimize symptoms such as pain and swelling, to prevent bone deformity (for example, bone erosions visible in X-rays), and to maintain day-to-day functioning.[95] This is primarily addressed with disease-modifying antirheumatic drugs (DMARDs); dosed physical activity; analgesics and physical therapy may be used to help manage pain.[7][5][6] RA should generally be treated with at least one specific anti-rheumatic medication[8] while combination therapies and corticosteroids are common in treatment.[96] The use of benzodiazepines (such as diazepam) to treat the pain is not recommended as it does not appear to help and is associated with risks.[97]
Lifestyle
Regular exercise is recommended as both safe and useful to maintain muscle strength and overall physical function.[98] Physical activity is beneficial for people with rheumatoid arthritis who experience fatigue,[99] although there was little to no evidence to suggest that exercise may have an impact on physical function in the long term, a study found that carefully dosed exercise has shown significant improvements in patients with RA.[6][100] Physical activity increases the production of synovial fluid, which lubricates the joints and reduces friction.[101] Moderate effects have been found for aerobic exercises and resistance training on cardiovascular fitness and muscle strength in RA. Furthermore, physical activity had no detrimental side effects like increased disease activity in any exercise dimension.[102] It is uncertain if eating or avoiding specific foods or other specific dietary measures help improve symptoms.[103] Occupational therapy has a positive role to play in improving functional ability in people with rheumatoid arthritis.[104] Weak evidence supports the use of wax baths (thermotherapy) to treat arthritis in the hands.[105]
Educational approaches that inform people about tools and strategies available to help them cope with rheumatoid arthritis may improve a person's psychological status and level of depression in the shorter-term.[106] The use of extra-depth shoes and molded insoles may reduce pain during weight-bearing activities such as walking.[107] Insoles may also prevent the progression of bunions.[107]
Disease-modifying agents
Disease-modifying antirheumatic drugs (DMARDs) are the primary treatment for RA.[8] They are a diverse collection of drugs, grouped by use and convention. They have been found to improve symptoms, decrease joint damage, and improve overall functional abilities.[8] DMARDs should be started early in the disease as they result in disease remission in approximately half of people and improved outcomes overall.[8]
The following drugs are considered DMARDs: methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, TNF inhibitors (certolizumab, adalimumab, infliximab and etanercept), abatacept, anakinra, and auranofin. Additionally, rituximab and tocilizumab are monoclonal antibodies and are also DMARDs.[8] Use of tocilizumab is associated with a risk of increased cholesterol levels.[108]
The most commonly used agent is methotrexate with other frequently used agents including sulfasalazine and leflunomide.[8] Leflunomide is effective when used from 6–12 months, with similar effectiveness to methotrexate when used for 2 years.[109] Sulfasalazine also appears to be most effective in the short-term treatment of rheumatoid arthritis.[110]
Hydroxychloroquine, in addition to its low toxicity profile, is considered effective for treatment of moderate RA treatment.[111]
Agents may be used in combination, however, people may experience greater side effects.[8][112] Methotrexate is the most important and useful DMARD and is usually the first treatment.[8][5][113] A combined approach with methotrexate and biologics improves ACR50, HAQ scores and RA remission rates.[114][47] This benefit from the combination of methotrexate with biologics occurs both when this combination is the initial treatment and when drugs are prescribed in a sequential or step-up manner.[47] Triple therapy consisting of methotrexate, sulfasalazine and hydroxychloroquine may also effectively control disease activity.[115] Adverse effects should be monitored regularly with toxicity including gastrointestinal, hematologic, pulmonary, and hepatic.[113] Side effects such as nausea, vomiting or abdominal pain can be reduced by taking folic acid.[116]
Rituximab combined with methotrexate appears to be more effective in improving symptoms compared to methotrexate alone.[117] Rituximab works by decreasing levels of B-cells (immune cell that is involved in inflammation). People taking rituximab had improved pain, function, reduced disease activity and reduced joint damage based on x-ray images. After 6 months, 21% more people had improvement in their symptoms using rituximab and methotrexate.[117]
Biological agents should generally be used only if methotrexate and other conventional agents are not effective after a trial of three months.[8] They are associated with a higher rate of serious infections as compared to other DMARDs.[118] Biological DMARD agents used to treat rheumatoid arthritis include: tumor necrosis factor alpha inhibitors (TNF inhibitors) such as infliximab; interleukin 1 blockers such as anakinra, monoclonal antibodies against B cells such as rituximab, interleukin 6 blockers such as tocilizumab, and T cell co-stimulation blockers such as abatacept. They are often used in combination with either methotrexate or leflunomide.[8][3] Biologic monotherapy or tofacitinib with methotrexate may improve ACR50, RA remission rates and function.[119][120] Abatacept should not be used at the same time as other biologics.[121] In those who are well controlled (low disease activity) on TNF inhibitors, decreasing the dose does not appear to affect overall function.[122] Discontinuation of TNF inhibitors (as opposed to gradually lowering the dose) by people with low disease activity may lead to increased disease activity and may affect remission, damage that is visible on an x-ray, and a person's function.[122] People should be screened for latent tuberculosis before starting any TNF inhibitor therapy to avoid reactivation of tuberculosis.[20]
TNF inhibitors and methotrexate appear to have similar effectiveness when used alone and better results are obtained when used together.[123] Golimumab is effective when used with methotraxate.[124] TNF inhibitors may have equivalent effectiveness with etanercept appearing to be the safest.[125] Injecting etanercept, in addition to methotrexate twice a week may improve ACR50 and decrease radiographic progression for up to 3 years.[126] Abatacept appears effective for RA with 20% more people improving with treatment than without but long term safety studies are yet unavailable.[127] Adalimumab slows the time for the radiographic progression when used for 52 weeks.[128] However, there is a lack of evidence to distinguish between the biologics available for RA.[129] Issues with the biologics include their high cost and association with infections including tuberculosis.[3] Use of biological agents may reduce fatigue.[130] The mechanism of how biologics reduce fatigue is unclear.[130]
Gold and cyclosporin
Sodium aurothiomalate, auranofin, and cyclosporin are less commonly used due to more common adverse effects.[8] However, cyclosporin was found to be effective in the progressive RA when used up to one year.[131]
Anti-inflammatory and analgesic agents
Glucocorticoids can be used in the short term and at the lowest dose possible for flare-ups and while waiting for slow-onset drugs to take effect.[8][3][132] Combination of glucocorticoids and conventional therapy has shown a decrease in rate of erosion of bones.[133] Steroids may be injected into affected joints during the initial period of RA, prior to the use of DMARDs or oral steroids.[134]
Non-NSAID drugs to relieve pain, like paracetamol may be used to help relieve the pain symptoms; they do not change the underlying disease.[5] The use of paracetamol may be associated with the risk of developing ulcers.[135]
NSAIDs reduce both pain and stiffness in those with RA but do not affect the underlying disease and appear to have no effect on people's long term disease course and thus are no longer first line agents.[3][136] NSAIDs should be used with caution in those with gastrointestinal, cardiovascular, or kidney problems.[137][138][139][135] Rofecoxib was withdrawn from the global market as its long-term use was associated to an increased risk of heart attacks and strokes.[140] Use of methotrexate together with NSAIDs is safe, if adequate monitoring is done.[141] COX-2 inhibitors, such as celecoxib, and NSAIDs are equally effective.[142][143] A 2004 Cochrane review found that people preferred NSAIDs over paracetamol.[144] However, it is yet to be clinically determined whether NSAIDs are more effective than paracetamol.[144]
The neuromodulator agents topical capsaicin may be reasonable to use in an attempt to reduce pain.[145] Nefopam by mouth and cannabis are not recommended as of 2012 as the risks of use appear to be greater than the benefits.[145]
Limited evidence suggests the use of weak oral opioids but the adverse effects may outweigh the benefits.[146]
Alternatively, physical therapy has been tested and shown as an effective aid in reducing pain in patients with RA. As most RA is detected early and treated aggressively, physical therapy plays more of a preventative and compensatory role, aiding in pain management alongside regular rheumatic therapy.[7]
Surgery
Especially for affected fingers, hands, and wrists, synovectomy may be needed to prevent pain or tendon rupture when drug treatment has failed. Severely affected joints may require joint replacement surgery, such as knee replacement. Postoperatively, physiotherapy is always necessary.[16]:1080, 1103 There is insufficient evidence to support surgical treatment on arthritic shoulders.[147]
Physiotherapy
For people with RA, physiotherapy may be used together with medical management.[148] This may include cold and heat application, electronic stimulation, and hydrotherapy.[148] Although medications improve symptoms of RA, muscle function is not regained when disease activity is controlled.[149]
Physiotherapy promotes physical activity. In RA, physical activity like exercise in the appropriate dosage (frequency, intensity, time, type, volume, progression) and physical activity promotion is effective in improving cardiovascular fitness, muscle strength, and maintaining a long term active lifestyle. In the short term, resistance exercises, with or without range of motion exercises, improve self-reported hand functions.[149] Physical activity promotion according to the public health recommendations should be an integral part of standard care for people with RA and other arthritic diseases.[6] Additionally, the combination of physical activities and cryotherapy show its efficacy on the disease activity and pain relief.[150] The combination of aerobic activity and cryotherapy may be an innovative therapeutic strategy to improve the aerobic capacity in arthritis patients and consequently reduce their cardiovascular risk while minimizing pain and disease activity.[150]
Compression gloves
Compression gloves are handwear designed to help prevent the occurrence of various medical disorders relating to blood circulation in the wrists and hands. They can be used to treat the symptoms of arthritis,[151] though the medical benefits may be limited.[152]
Alternative medicine
In general, there is not enough evidence to support any complementary health approaches for RA, with safety concerns for some of them. Some mind and body practices and dietary supplements may help people with symptoms and therefore may be beneficial additions to conventional treatments, but there is not enough evidence to draw conclusions.[153] A systematic review of CAM modalities (excluding fish oil) found that " The available evidence does not support their current use in the management of RA."[154] Studies showing beneficial effects in RA on a wide variety of CAM modalities are often affected by publication bias and are generally not high quality evidence such as randomized controlled trials (RCTs).[155]
A 2005 Cochrane review states that low level laser therapy can be tried to improve pain and morning stiffness due to rheumatoid arthritis as there are few side-effects.[156]
There is limited evidence that tai chi might improve the range of motion of a joint in persons with rheumatoid arthritis.[157][158] The evidence for acupuncture is inconclusive[159] with it appearing to be equivalent to sham acupuncture.[160]
A Cochrane review in 2002 showed some benefits of the electrical stimulation as a rehabilitation intervention to improve the power of the hand grip and help to resist fatigue.[161] D‐penicillamine may provide similar benefits as DMARDs but it is also highly toxic.[162] Low-quality evidence suggests the use of therapeutic ultrasound on arthritic hands.[163] Potential benefits include increased grip strength, reduced morning stiffness and number of swollen joints.[163] There is tentative evidence of benefit of transcutaneous electrical nerve stimulation (TENS) in RA.[164] Acupuncture‐like TENS (AL-TENS) may decrease pain intensity and improve muscle power scores.[164]
Low-quality evidence suggests people with active RA may benefit from assistive technology.[165] This may include less discomfort and difficulty such as when using an eye drop device.[165] Balance training is of unclear benefits.[166]
Dietary supplements
Fatty acids
There has been a growing interest in the role of long-chain omega-3 polyunsaturated fatty acids to reduce inflammation and alleviate the symptoms of RA. Metabolism of omega-3 polyunsaturated fatty acids produces docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which inhibits pro-inflammatory eicosanoids and cytokines (TNF-a, IL-1b and IL-6), decreasing both lymphocyte proliferation and reactive oxygen species.[167][168] These studies showed evidence for significant clinical improvements on RA in inflammatory status and articular index. Gamma-linolenic acid, an omega-6 fatty acid, may reduce pain, tender joint count and stiffness, and is generally safe.[169] For omega-3 polyunsaturated fatty acids (found in fish oil, flax oil and hemp oil), a meta-analysis reported a favorable effect on pain, although confidence in the effect was considered moderate. The same review reported less inflammation but no difference in joint function.[170] A review examined the effect of marine oil omega-3 fatty acids on pro-inflammatory eicosanoid concentrations; leukotriene4 (LTB4) was lowered in people with rheumatoid arthritis but not in those with non-autoimmune chronic diseases.[171] Fish consumption has no association with RA.[172] A fourth review limited inclusion to trials in which people eat ≥2.7 g/day for more than three months. Use of pain relief medication was decreased, but improvements in tender or swollen joints, morning stiffness and physical function were not changed.[173] Collectively, the current evidence is not strong enough to determine that supplementation with omega-3 fatty acids or regular consumption of fish are effective treatments for rheumatoid arthritis.[170][171][172][173]
Herbal
The American College of Rheumatology states that no herbal medicines have health claims supported by high-quality evidence and thus they do not recommend their use.[174] There is no scientific basis to suggest that herbal supplements advertised as "natural" are safer for use than conventional medications as both are chemicals. Herbal medications, although labelled "natural", may be toxic or fatal if consumed.[174] Due to the false belief that herbal supplements are always safe, there is sometimes a hesitancy to report their use which may increase the risk of adverse reactions.[155]
Pregnancy
More than 75% of women with rheumatoid arthritis have symptoms improve during pregnancy but might have symptoms worsen after delivery.[20] Methotrexate and leflunomide are teratogenic (harmful to foetus) and not used in pregnancy. It is recommended women of childbearing age should use contraceptives to avoid pregnancy and to discontinue its use if pregnancy is planned.[95][113] Low dose of prednisolone, hydroxychloroquine and sulfasalazine are considered safe in pregnant women with rheumatoid arthritis. Prednisolone should be used with caution as the side effects include infections and fractures.[175]
Vaccinations
People with RA have an increased risk of infections and mortality and recommended vaccinations can reduce these risks.[176] The inactivated influenza vaccine should be received annually.[177] The pneumococcal vaccine should be administered twice for people under the age 65 and once for those over 65.[178] Lastly, the live-attenuated zoster vaccine should be administered once after the age 60, but is not recommended in people on a tumor necrosis factor alpha blocker.[179]
Prognosis

The course of the disease varies greatly. Some people have mild short-term symptoms, but in most the disease is progressive for life. Around 25% will have subcutaneous nodules (known as rheumatoid nodules);[181] this is associated with a poor prognosis.[182]
Prognostic factors
Poor prognostic factors include,
- Persistent synovitis
- Early erosive disease
- Extra-articular findings (including subcutaneous rheumatoid nodules)
- Positive serum RF findings
- Positive serum anti-CCP autoantibodies
- Positive serum 14-3-3η (YWHAH) levels above 0.5 ng/ml [183][184]
- Carriership of HLA-DR4 "Shared Epitope" alleles
- Family history of RA
- Poor functional status
- Socioeconomic factors[185]
- Elevated acute phase response (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP])
- Increased clinical severity.
- Distance from primary care and specialist care in rural communities[185]
Mortality
RA reduces lifespan on average from three to twelve years.[95] Young age at onset, long disease duration, the presence of other health problems, and characteristics of severe RA – such as poor functional ability or overall health status, a lot of joint damage on x-rays, the need for hospitalisation or involvement of organs other than the joints – have been shown to associate with higher mortality.[186] Positive responses to treatment may indicate a better prognosis. A 2005 study by the Mayo Clinic noted that individuals with RA have a doubled risk of heart disease,[187] independent of other risk factors such as diabetes, excessive alcohol use, and elevated cholesterol, blood pressure and body mass index. The mechanism by which RA causes this increased risk remains unknown; the presence of chronic inflammation has been proposed as a contributing factor.[188] It is possible that the use of new biologic drug therapies extend the lifespan of people with RA and reduce the risk and progression of atherosclerosis.[189] This is based on cohort and registry studies, and still remains hypothetical. It is still uncertain whether biologics improve vascular function in RA or not. There was an increase in total cholesterol and HDLc levels and no improvement of the atherogenic index.[190]
Epidemiology
RA affects 0.5–1% of adults in the developed world with between 5 and 50 per 100,000 people newly developing the condition each year.[3] In 2010 it resulted in about 49,000 deaths globally.[191]
Onset is uncommon under the age of 15 and from then on the incidence rises with age until the age of 80. Women are affected three to five times as often as men.[20]
The age at which the disease most commonly starts is in women between 40 and 50 years of age, and for men somewhat later.[192] RA is a chronic disease, and although rarely, a spontaneous remission may occur, the natural course is almost invariably one of the persistent symptoms, waxing and waning in intensity, and a progressive deterioration of joint structures leading to deformations and disability.[citation needed]
There is an association between periodontitis and rheumatoid arthritis (RA), hypothesised to lead to enhanced generation of RA-related autoantibodies. Oral bacteria that invade the blood may also contribute to chronic inflammatory responses and generation of autoantibodies.[193]
History
The first recognized description of RA in modern medicine was in 1800 by the French physician Augustin Jacob Landré-Beauvais (1772–1840) who was based in the famed Salpêtrière Hospital in Paris.[12] The name "rheumatoid arthritis" itself was coined in 1859 by British rheumatologist Alfred Baring Garrod.[194]
The art of Peter Paul Rubens may possibly depict the effects of RA. In his later paintings, his rendered hands show, in the opinion of some physicians, increasing deformity consistent with the symptoms of the disease.[195][196] RA appears to some to have been depicted in 16th-century paintings.[197] However, it is generally recognized in art historical circles that the painting of hands in the 16th and 17th century followed certain stylized conventions, most clearly seen in the Mannerist movement. It was conventional, for instance, to show the upheld right hand of Christ in what now appears a deformed posture. These conventions are easily misinterpreted as portrayals of disease.[citation needed]
Historic (though not necessarily effective) treatments for RA have also included: rest, ice, compression and elevation, apple diet, nutmeg, some light exercise every now and then, nettles, bee venom, copper bracelets, rhubarb diet, extractions of teeth, fasting, honey, vitamins, insulin, magnets, and electroconvulsive therapy (ECT).[198]
Etymology
Rheumatoid arthritis is derived from the Greek word ῥεύμα-rheuma (nom.), ῥεύματος-rheumatos (gen.) ("flow, current"). The suffix -oid ("resembling") gives the translation as joint inflammation that resembles rheumatic fever. Rhuma which means watery discharge might refer to the fact that the joints are swollen or that the disease may be made worse by wet weather.[13]
Research
Meta-analysis found an association between periodontal disease and RA, but the mechanism of this association remains unclear.[199] Two bacterial species associated with periodontitis are implicated as mediators of protein citrullination in the gums of people with RA.[3]
Vitamin D deficiency is more common in people with rheumatoid arthritis than in the general population.[200][201] However, whether vitamin D deficiency is a cause or a consequence of the disease remains unclear.[202] One meta-analysis found that vitamin D levels are low in people with rheumatoid arthritis and that vitamin D status correlates inversely with prevalence of rheumatoid arthritis, suggesting that vitamin D deficiency is associated with susceptibility to rheumatoid arthritis.[203]
The fibroblast-like synoviocytes have a prominent role in the pathogenic processes of the rheumatic joints, and therapies that target these cells are emerging as promising therapeutic tools, raising hope for future applications in rheumatoid arthritis.[17]
Possible links with intestinal barrier dysfunction are investigated.[204]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 "Handout on Health: Rheumatoid Arthritis". August 2014. http://www.niams.nih.gov/health_info/Rheumatic_Disease/default.asp.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Rheumatoid arthritis: diagnosis and management". The American Journal of Medicine 120 (11): 936–939. November 2007. doi:10.1016/j.amjmed.2007.04.005. PMID 17976416.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 "Rheumatoid arthritis". Lancet 388 (10055): 2023–2038. October 2016. doi:10.1016/S0140-6736(16)30173-8. PMID 27156434. http://eprints.gla.ac.uk/131249/1/131249.pdf.
- ↑ "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet 388 (10053): 1459–1544. October 2016. doi:10.1016/S0140-6736(16)31012-1. PMID 27733281.
- ↑ 5.0 5.1 5.2 5.3 "Rheumatoid arthritis in adults: management: recommendations: Guidance and guidelines". NICE. December 2015. https://www.nice.org.uk/guidance/CG79/chapter/Recommendations.
- ↑ 6.0 6.1 6.2 6.3 "Effects of exercise and physical activity promotion: meta-analysis informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis". RMD Open 4 (2): e000713. 2018-12-04. doi:10.1136/rmdopen-2018-000713. PMID 30622734.
- ↑ 7.0 7.1 7.2 "Effects of rehabilitation for pain relief in patients with rheumatoid arthritis: a systematic review". Journal of Physical Therapy Science 28 (1): 304–308. January 2016. doi:10.1589/jpts.28.304. PMID 26957779.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 "2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis". Arthritis & Rheumatology 68 (1): 1–26. January 2016. doi:10.1002/art.39480. PMID 26545940.
- ↑ "Adverse effects of biologics: a network meta-analysis and Cochrane overview". The Cochrane Database of Systematic Reviews 2011 (2): CD008794. February 2011. doi:10.1002/14651858.CD008794.pub2. PMID 21328309.
- ↑ "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet 388 (10053): 1545–1602. October 2016. doi:10.1016/S0140-6736(16)31678-6. PMID 27733282.
- ↑ "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet 385 (9963): 117–171. January 2015. doi:10.1016/S0140-6736(14)61682-2. PMID 25530442.
- ↑ 12.0 12.1 La goutte asthénique primitive (doctoral thesis). Paris. 1800. reproduced in "The first description of rheumatoid arthritis. Unabridged text of the doctoral dissertation presented in 1800". Joint, Bone, Spine 68 (2): 130–143. March 2001. doi:10.1016/S1297-319X(00)00247-5. PMID 11324929.
- ↑ 13.0 13.1 The Hospital for Special Surgery Rheumatoid Arthritis Handbook Everything You Need to Know. New York: John Wiley & Sons. 2002. pp. 32. ISBN 978-0-471-22334-4. https://books.google.com/books?id=akacOyQrnKkC&pg=PA32.
- ↑ "Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years". Annals of the Rheumatic Diseases 62 (8): 722–727. August 2003. doi:10.1136/ard.62.8.722. PMID 12860726.
- ↑ "Burden of disease in treated rheumatoid arthritis patients: going beyond the joint". Seminars in Arthritis and Rheumatism 43 (4): 479–488. February 2014. doi:10.1016/j.semarthrit.2013.08.004. PMID 24080116.
- ↑ 16.0 16.1 16.2 16.3 Davidson's principles and practice of medicine (22nd ed.). Churchill Livingstone/Elsevier. 2014. ISBN 978-0-7020-5035-0.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 "Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes". Nature Reviews. Rheumatology 16 (6): 316–333. June 2020. doi:10.1038/s41584-020-0413-5. PMID 32393826.
- ↑ "Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know". Journal of the Royal Society of Medicine 97 (9): 421–424. September 2004. doi:10.1177/014107680409700903. PMID 15340020.
- ↑ "Treatment of rheumatoid arthritis". American Journal of Health-System Pharmacy 63 (24): 2451–2465. December 2006. doi:10.2146/ajhp050514. PMID 17158693.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 20.8 Harrison's Principles of Internal Medicine (18th ed.). United States: McGraw Hill. 2012. pp. 2738. ISBN 978-0-07-174889-6.
- ↑ "Extra-articular rheumatoid arthritis". Current Opinion in Rheumatology 25 (3): 360–366. May 2013. doi:10.1097/bor.0b013e32835f693f. PMID 23425964.
- ↑ "The rheumatoid nodule". Arthritis and Rheumatism 33 (6): 761–767. June 1990. doi:10.1002/art.1780330601. PMID 2194460.
- ↑ "Systemic rheumatoid vasculitis: a review". Seminars in Arthritis and Rheumatism 36 (2): 88–98. October 2006. doi:10.1016/j.semarthrit.2006.04.006. PMID 17023257.
- ↑ 24.0 24.1 Alopecia Areata. 2018. doi:10.1007/978-3-319-72134-7. ISBN 978-3-319-72133-0.
- ↑ "Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern". Chest 136 (5): 1397–1405. November 2009. doi:10.1378/chest.09-0444. PMID 19892679.
- ↑ "Rheumatoid pleural effusion". Seminars in Arthritis and Rheumatism 35 (6): 368–378. June 2006. doi:10.1016/j.semarthrit.2006.03.002. PMID 16765714.
- ↑ "The mortality of rheumatoid arthritis". Arthritis and Rheumatism 37 (4): 481–494. April 1994. doi:10.1002/art.1780370408. PMID 8147925.
- ↑ "Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies". Arthritis and Rheumatism 59 (12): 1690–1697. December 2008. doi:10.1002/art.24092. PMID 19035419.
- ↑ "The Prevalence of Atherosclerosis in Those with Inflammatory Connective Tissue Disease by Race, Age, and Traditional Risk Factors". Scientific Reports 6: 20303. February 2016. doi:10.1038/srep20303. PMID 26842423. Bibcode: 2016NatSR...620303A.
- ↑ 30.0 30.1 "Evaluating cardiovascular risk in rheumatoid arthritis". Journal of Musculoskeletal Medicine 26 (8): 481–494. 2009. http://www.musculoskeletalnetwork.com/rheumatoid-arthritis/content/article/1145622/1433094.
- ↑ "Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature". The American Journal of Medicine 116 (Suppl 7A): 50S–57S. April 2004. doi:10.1016/j.amjmed.2003.12.012. PMID 15050886.
- ↑ "Role of Platelets in Osteoarthritis-Updated Systematic Review and Meta-Analysis on the Role of Platelet-Rich Plasma in Osteoarthritis". Cells 11 (7): 1080. March 2022. doi:10.3390/cells11071080. PMID 35406644.
- ↑ "The role of the circadian clock in rheumatoid arthritis". Arthritis Res Ther 15 (1): 205. February 2013. doi:10.1186/ar4146. PMID 23427807.
- ↑ "[Renal manifestations in rheumatic diseases]". Der Internist 48 (8): 779–785. August 2007. doi:10.1007/s00108-007-1887-9. PMID 17571244.
- ↑ "Secondary Membranous Nephropathy. A Narrative Review". Frontiers in Medicine 7: 611317. 2020. doi:10.3389/fmed.2020.611317. PMID 33344486.
- ↑ "Episcleritis". StatPearls. Treasure Island (FL): StatPearls Publishing. January 2020. http://www.ncbi.nlm.nih.gov/books/nbk534796/.
- ↑ "Natural and iatrogenic ocular manifestations of rheumatoid arthritis: a systematic review". International Ophthalmology 42 (2): 689–711. February 2022. doi:10.1007/s10792-021-02058-8. PMID 34802085.
- ↑ 38.0 38.1 "Liver involvement in subjects with rheumatic disease". Arthritis Research & Therapy 13 (3): 226. June 2011. doi:10.1186/ar3319. PMID 21722332.
- ↑ "Rheumatoid arthritis of the cervical spine--clinical considerations". Bulletin of the NYU Hospital for Joint Diseases 69 (2): 136–148. 2011. PMID 22035393. http://hjdbulletin.org/files/archive/pdfs/222.pdf.
- ↑ "Osteoporosis, inflammation and ageing". Immunity & Ageing 2: 14. November 2005. doi:10.1186/1742-4933-2-14. PMID 16271143.
- ↑ "An Overview of Glucocorticoid-Induced Osteoporosis.". Endotext [Internet].. South Dartmouth (MA): MDText.com, Inc.. March 2022. https://www.ncbi.nlm.nih.gov/sites/books/NBK278968/.
- ↑ "Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis". Arthritis and Rheumatism 54 (3): 692–701. March 2006. doi:10.1002/art.21675. PMID 16508929.
- ↑ "Incidence of lymphoma in a large primary care derived cohort of cases of inflammatory polyarthritis". Annals of the Rheumatic Diseases 65 (5): 617–622. May 2006. doi:10.1136/ard.2005.044784. PMID 16249224.
- ↑ "Rheumatoid arthritis, TNF inhibitors, and non-melanoma skin cancer". BMJ 352: i472. January 2016. doi:10.1136/bmj.i472. PMID 26822198.
- ↑ "Periodontitis in systemic rheumatic diseases". Nature Reviews. Rheumatology 5 (4): 218–224. April 2009. doi:10.1038/nrrheum.2009.28. PMID 19337286.
- ↑ "Nutritional management of rheumatoid arthritis: a review of the evidence". Journal of Human Nutrition and Dietetics 16 (2): 97–109. April 2003. doi:10.1046/j.1365-277x.2003.00423.x. PMID 12662368.
- ↑ 47.0 47.1 47.2 47.3 Donahue, Katrina E.; Gartlehner, Gerald; Schulman, Elizabeth R.; Jonas, Beth; Coker-Schwimmer, Emmanuel; Patel, Sheila V.; Weber, Rachel Palmieri; Lohr, Kathleen N. et al. (2018). Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update. AHRQ Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US). http://www.ncbi.nlm.nih.gov/books/NBK524950/.
- ↑ 48.0 48.1 Musculosketal Disorders-Davidson's Principle of Internal Medicine (20th ed.). Elsevier. pp. 1100–1106.
- ↑ "Genetics of rheumatoid arthritis contributes to biology and drug discovery". Nature 506 (7488): 376–381. February 2014. doi:10.1038/nature12873. PMID 24390342. Bibcode: 2014Natur.506..376..
- ↑ "Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis". Nature Genetics 44 (3): 291–296. January 2012. doi:10.1038/ng.1076. PMID 22286218.
- ↑ "PTPN22 1858 C/T polymorphism is associated with alteration of cytokine profiles as a potential pathogenic mechanism in rheumatoid arthritis". Immunology Letters 216: 106–113. December 2019. doi:10.1016/j.imlet.2019.10.010. PMID 31669381.
- ↑ "Immunopathogenesis of Rheumatoid Arthritis". Immunity 46 (2): 183–196. February 2017. doi:10.1016/j.immuni.2017.02.006. PMID 28228278.(Subscription content?)
- ↑ 53.0 53.1 "Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies". Annals of the Rheumatic Diseases 69 (1): 70–81. January 2010. doi:10.1136/ard.2008.096487. PMID 19174392. http://www.lib.kobe-u.ac.jp/repository/90001457.pdf. Retrieved 2018-04-20.(Subscription content?)
- ↑ "Environmental influences on risk for rheumatoid arthritis". Current Opinion in Rheumatology 21 (3): 279–283. May 2009. doi:10.1097/BOR.0b013e32832a2e16. PMID 19318947.(Subscription content?)
- ↑ "Silica, Silicosis, and Autoimmunity". Frontiers in Immunology 7: 97. 11 March 2016. doi:10.3389/fimmu.2016.00097. PMID 27014276.
- ↑ "Do self-perpetuating B lymphocytes drive human autoimmune disease?". Immunology 97 (2): 188–196. June 1999. doi:10.1046/j.1365-2567.1999.00772.x. PMID 10447731.(Subscription content?)
- ↑ "Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same?". Seminars in Arthritis and Rheumatism 46 (3): 291–304. December 2016. doi:10.1016/j.semarthrit.2016.05.012. PMID 27388027.
- ↑ "A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis". Arthritis and Rheumatism 50 (10): 3085–3092. October 2004. doi:10.1002/art.20553. PMID 15476204. http://rsp.ima-press.net/rsp/article/view/1939.(Subscription content?)
- ↑ "Diagnosis, prognosis and classification of early arthritis: results of a systematic review informing the 2016 update of the EULAR recommendations for the management of early arthritis". RMD Open 3 (1): e000406. 2017. doi:10.1136/rmdopen-2016-000406. PMID 28155923.
- ↑ 60.0 60.1 "Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review". Journal of Autoimmunity 57 (6): 1–13. February 2015. doi:10.1016/j.jaut.2014.12.002. PMID 25578468.(Subscription content?)
- ↑ Relevance of the lectin pathway of complement in rheumatic diseases. 56. Elsevier. 2012. 105–153. doi:10.1016/B978-0-12-394317-0.00012-1. ISBN 978-0-12-394317-0.(Subscription content?)
- ↑ 62.0 62.1 "Granzyme K+ CD8 T cells form a core population in inflamed human tissue". Science Translational Medicine 14 (649): eabo0686. June 2022. doi:10.1126/scitranslmed.abo0686. PMID 35704599.
- ↑ "The pathogenic role of angiogenesis in rheumatoid arthritis". Angiogenesis 18 (4): 433–448. October 2015. doi:10.1007/s10456-015-9477-2. PMID 26198292.
- ↑ "Calprotectin as a biomarker for rheumatoid arthritis: a systematic review". The Journal of Rheumatology 42 (5): 760–770. May 2015. doi:10.3899/jrheum.140628. PMID 25729036.
- ↑ "Inherent low Erk and p38 activity reduce Fas Ligand expression and degranulation in T helper 17 cells leading to activation induced cell death resistance". Oncotarget 7 (34): 54339–54359. August 2016. doi:10.18632/oncotarget.10913. PMID 27486885.
- ↑ "Denosumab: targeting the RANKL pathway to treat rheumatoid arthritis". Expert Opinion on Biological Therapy 17 (1): 119–128. January 2017. doi:10.1080/14712598.2017.1263614. PMID 27871200.(Subscription content?)
- ↑ "Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs". Arthritis Research & Therapy 8 (3): R76. 2006. doi:10.1186/ar1943. PMID 16646983.
- ↑ "Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis". Rheumatology 57 (1): 49–58. January 2018. doi:10.1093/rheumatology/kex036. PMID 28340066.
- ↑ "Modern ultrasound methods yield stronger arthritis work-up". Diagnostic Imaging 32. Apr 29, 2010. http://www.diagnosticimaging.com/ultrasound/modern-ultrasound-methods-yield-stronger-arthritis-work. Retrieved October 21, 2018.
- ↑ "Rheumatoid factors: what's new?". Rheumatology 45 (4): 379–385. April 2006. doi:10.1093/rheumatology/kei228. PMID 16418203.(Subscription content?)
- ↑ "Importance of 14-3-3eta, anti-CarP, and anti-Sa in the diagnosis of seronegative rheumatoid arthritis". Turkish Journal of Medical Sciences 49 (5): 1498–1502. October 2019. doi:10.3906/sag-1812-137. PMID 31651120.
- ↑ "Anti-CCP antibodies: the past, the present and the future". Nature Reviews. Rheumatology 7 (7): 391–398. June 2011. doi:10.1038/nrrheum.2011.76. PMID 21647203.(Subscription content?)
- ↑ "Anti-MCV Antibody Test for the Diagnosis of Rheumatoid Arthritis Using a POCT-Immunoassay". American College of Rheumatology, 2008 Annual Scientific Meeting, Poster Presentation. http://acr.confex.com/acr/2008/webprogram/Paper2009.html.
- ↑ "Does anti-mutated citrullinated vimentin have additional value as a serological marker in the diagnostic and prognostic investigation of patients with rheumatoid arthritis? A systematic review". Annals of the Rheumatic Diseases 69 (2): 337–344. February 2010. doi:10.1136/ard.2008.103283. PMID 19289382. http://ard.bmj.com/cgi/content/short/ard.2008.103283v1.(Subscription content?)
- ↑ "The role of 14-3-3 η as a biomarker in rheumatoid arthritis". Rheumatology and Immunology Research. 2 (2): 87–90. June 2021. doi:10.2478/rir-2021-0012. PMID 36465971.
- ↑ "Autoimmune Conditions in 235 Hemochromatosis Probands with HFE C282Y Homozygosity and Their First-Degree Relatives". Journal of Immunology Research 2015: 453046. 2015. doi:10.1155/2015/453046. PMID 26504855.
- ↑ 77.0 77.1 "2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative". Annals of the Rheumatic Diseases 69 (9): 1580–1588. September 2010. doi:10.1136/ard.2010.138461. PMID 20699241. https://deepblue.lib.umich.edu/bitstream/2027.42/78045/1/27584_ftp.pdf.
- ↑ "The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis". Arthritis and Rheumatism 31 (3): 315–34. March 1988. doi:10.1002/art.1780310302. PMID 3358796.
- ↑ 79.0 79.1 Berkow R, ed (1992). The Merck Manual (16th ed.). Merck Publishing Group. pp. 1307–1308. ISBN 978-0-911910-16-2. https://archive.org/details/merckmanualofdia16berk.(Subscription content?)
- ↑ "Response to: 'Correspondence on 'EULAR definition of difficult-to-treat rheumatoid arthritis by Novella-Navarro et al". Annals of the Rheumatic Diseases 82 (3): annrheumdis–2020–219535. December 2020. doi:10.1136/annrheumdis-2020-219535. PMID 33277239.
- ↑ "Recent advances in the genetics of rheumatoid arthritis". Current Opinion in Rheumatology 22 (2): 109–118. March 2010. doi:10.1097/bor.0b013e328336474d. PMID 20075733.
- ↑ "Challenges in the treatment of Rheumatoid Arthritis". Autoimmunity Reviews 18 (7): 706–713. July 2019. doi:10.1016/j.autrev.2019.05.007. PMID 31059844.
- ↑ Conigliaro, Paola; Ciccacci, Cinzia; Politi, Cristina; Triggianese, Paola; Rufini, Sara; Kroegler, Barbara; Perricone, Carlo; Latini, Andrea et al. (2017-01-20). Ahuja, Sunil K. ed. "Polymorphisms in STAT4, PTPN2, PSORS1C1 and TRAF3IP2 Genes Are Associated with the Response to TNF Inhibitors in Patients with Rheumatoid Arthritis" (in en). PLOS ONE 12 (1): e0169956. doi:10.1371/journal.pone.0169956. ISSN 1932-6203. PMID 28107378. Bibcode: 2017PLoSO..1269956C.
- ↑ "Association of biomarkers of inflammation and HLA-DRB1 gene locus with risk of developing rheumatoid arthritis in females". Rheumatology International 39 (12): 2147–2157. December 2019. doi:10.1007/s00296-019-04429-y. PMID 31451934.
- ↑ "The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis". Arthritis and Rheumatism 30 (11): 1205–1213. November 1987. doi:10.1002/art.1780301102. PMID 2446635.
- ↑ "Association of rheumatoid arthritis with HLA-DR1 and HLA-DR4 in Hungary". Annals of the New York Academy of Sciences 1051 (1): 263–270. June 2005. doi:10.1196/annals.1361.067. PMID 16126967. Bibcode: 2005NYASA1051..263K.
- ↑ "Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFα". Arthritis Research & Therapy 17 (1): 49. March 2015. doi:10.1186/s13075-015-0555-z. PMID 25860297.
- ↑ 88.0 88.1 "Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis". Arthritis and Rheumatism 38 (1): 44–48. January 1995. doi:10.1002/art.1780380107. PMID 7818570. http://www.pharmacoeconomics.ru/jour/article/view/135.(Subscription content?)
- ↑ Kelly, Janis (22 February 2005) DAS28 not always a reliable indicator of treatment effect in RA , Medscape Medical News.
- ↑ "Correlación entre la actividad clínica por DAS-28 y ecografía en pacientes con artritis reumatoide" (in es). Revista Colombiana de Reumatología 23 (3): 159–169. July–September 2016. doi:10.1016/j.rcreu.2016.05.002.
- ↑ "Tools for monitoring remission in rheumatoid arthritis: any will do, let's just pick one and start measuring". Arthritis Research & Therapy 15 (1): 104. January 2013. doi:10.1186/ar4139. PMID 23374997.(Subscription content?)
- ↑ "The Stanford Health Assessment Questionnaire: dimensions and practical applications". Health and Quality of Life Outcomes 1: 20. June 2003. doi:10.1186/1477-7525-1-20. PMID 12831398.(Subscription content?)
- ↑ "American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis". Arthritis and Rheumatism 59 (6): 762–784. June 2008. doi:10.1002/art.23721. PMID 18512708.
- ↑ "Comparative Effectiveness of Combining MTX with Biologic Drug Therapy Versus Either MTX or Biologics Alone for Early Rheumatoid Arthritis in Adults: a Systematic Review and Network Meta-analysis". Journal of General Internal Medicine 34 (10): 2232–2245. October 2019. doi:10.1007/s11606-019-05230-0. PMID 31388915.
- ↑ 95.0 95.1 95.2 "Diagnosis and management of rheumatoid arthritis". American Family Physician 84 (11): 1245–1252. December 2011. PMID 22150658.
- ↑ Donahue, Katrina E.; Gartlehner, Gerald; Schulman, Elizabeth R.; Jonas, Beth; Coker-Schwimmer, Emmanuel; Patel, Sheila V.; Weber, Rachel Palmieri; Lohr, Kathleen N. et al. (2018-07-16). "Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update". Effective Health Care Program. doi:10.23970/ahrqepccer211. https://effectivehealthcare.ahrq.gov/topics/rheumatoid-arthritis-medicine-update/final-report-update-2018.
- ↑ "Muscle relaxants for pain management in rheumatoid arthritis". The Cochrane Database of Systematic Reviews 1: CD008922. January 2012. doi:10.1002/14651858.CD008922.pub2. PMID 22258993.
- ↑ "Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2009 (4): CD006853. October 2009. doi:10.1002/14651858.CD006853.pub2. PMID 19821388.
- ↑ "Non-pharmacological interventions for fatigue in rheumatoid arthritis". The Cochrane Database of Systematic Reviews (8): CD008322. August 2013. doi:10.1002/14651858.CD008322.pub2. PMID 23975674.
- ↑ "Exercise for rheumatoid arthritis of the hand". The Cochrane Database of Systematic Reviews 2018 (7): CD003832. July 2018. doi:10.1002/14651858.cd003832.pub3. PMID 30063798.
- ↑ Jabeen, Attiya (16 August 2023). "The Benefits of Exercise in Rheumatoid Arthritis: A Comprehensive Guide". Attiya Jabeen. https://rheumatologydelaware.com/benefits-exercise-in-rheumatoid-arthritis/.
- ↑ "Effects of exercise and physical activity promotion: meta-analysis informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis". RMD Open 4 (2): e000713. December 2018. doi:10.1136/rmdopen-2018-000713. PMID 30622734.
- ↑ "Dietary interventions for rheumatoid arthritis". The Cochrane Database of Systematic Reviews (1): CD006400. January 2009. doi:10.1002/14651858.CD006400.pub2. PMID 19160281.
- ↑ "Occupational therapy for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2004 (1): CD003114. 2004. doi:10.1002/14651858.CD003114.pub2. PMID 14974005. PMC 7017227. https://repository.ubn.ru.nl/bitstream/2066/58846/1/58846.pdf.
- ↑ "Thermotherapy for treating rheumatoid arthritis". The Cochrane Database of Systematic Reviews (2): CD002826. 2002-04-22. doi:10.1002/14651858.cd002826. PMID 12076454.
- ↑ "Patient education for adults with rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2009 (2): CD003688. 2003-04-22. doi:10.1002/14651858.cd003688. PMID 12804484. https://research.utwente.nl/en/publications/patient-education-for-adults-with-rheumatoid-arthritis-review(6d6c077a-30a1-4b7c-8021-f72f1a8b6a5c).html.
- ↑ 107.0 107.1 "Splints/orthoses in the treatment of rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2001 (1): CD004018. 2001-10-23. doi:10.1002/14651858.cd004018. PMID 12535502.
- ↑ "Tocilizumab for rheumatoid arthritis". Cochrane Database of Systematic Reviews. Advances in Experimental Medicine and Biology. 764. John Wiley & Sons. 2010-07-07. pp. 151–158. doi:10.1002/14651858.cd008331.pub2.
- ↑ "Leflunomide for treating rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2002 (1): CD002047. 2003. doi:10.1002/14651858.CD002047. PMID 12535423.
- ↑ "Sulfasalazine for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 1998 (2): CD000958. 1998-04-27. doi:10.1002/14651858.cd000958. PMID 10796400.
- ↑ "Antimalarials for treating rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2010 (4): CD000959. 2000. doi:10.1002/14651858.CD000959. PMID 11034691.
- ↑ "Methotrexate monotherapy versus methotrexate combination therapy with non-biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2015 (4): CD008495. April 2010. doi:10.1002/14651858.cd008495. PMID 20393970.
- ↑ 113.0 113.1 113.2 Pharmacotherapy: a Pathophysiologic Approach (7th ed.). New York: McGraw-Hill. 2008. ISBN 978-0-07-147899-1.
- ↑ "Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis". The Cochrane Database of Systematic Reviews 5 (5): CD012657. May 2017. doi:10.1002/14651858.cd012657. PMID 28481462.
- ↑ "Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis: A network meta-analysis". The Cochrane Database of Systematic Reviews 2016 (8): CD010227. August 2016. doi:10.1002/14651858.cd010227.pub2. PMID 27571502.
- ↑ "Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 5 (5): CD000951. May 2013. doi:10.1002/14651858.CD000951.pub2. PMID 23728635.
- ↑ 117.0 117.1 "Rituximab for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 1: CD007356. January 2015. doi:10.1002/14651858.CD007356.pub2. PMID 25603545.
- ↑ "Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis". Lancet 386 (9990): 258–265. July 2015. doi:10.1016/S0140-6736(14)61704-9. PMID 25975452.
- ↑ "Biologic or tofacitinib monotherapy for rheumatoid arthritis in people with traditional disease-modifying anti-rheumatic drug (DMARD) failure: a Cochrane Systematic Review and network meta-analysis (NMA)". The Cochrane Database of Systematic Reviews 2016 (11): CD012437. November 2016. doi:10.1002/14651858.cd012437. PMID 27855242.
- ↑ "Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta-analysis". The Cochrane Database of Systematic Reviews 2017 (3): CD012591. March 2017. doi:10.1002/14651858.cd012591. PMID 28282491.
- ↑ "Abatacept for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2009 (4): CD007277. October 2009. doi:10.1002/14651858.CD007277.pub2. PMID 19821401.
- ↑ 122.0 122.1 "Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity". The Cochrane Database of Systematic Reviews 5 (6): CD010455. May 2019. doi:10.1002/14651858.CD010455.pub3. PMID 31125448.
- ↑ Donahue, Katrina E.; Gartlehner, Gerald; Schulman, Elizabeth R.; Jonas, Beth; Coker-Schwimmer, Emmanuel; Patel, Sheila V.; Weber, Rachel Palmieri; Lohr, Kathleen N. et al. (2018-07-16) (in en). Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update (Report). Agency for Healthcare Research and Quality (AHRQ). doi:10.23970/ahrqepccer211. https://doi.org/10.23970/AHRQEPCCER211.
- ↑ "Golimumab for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2010 (1): CD008341. January 2010. doi:10.1002/14651858.CD008341. PMID 20091667.
- ↑ "Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis". PLOS ONE 7 (1): e30275. 2012. doi:10.1371/journal.pone.0030275. PMID 22272322. Bibcode: 2012PLoSO...730275A.
- ↑ "Etanercept for the treatment of rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2014 (5): CD004525. May 2013. doi:10.1002/14651858.cd004525.pub2. PMID 23728649.
- ↑ "Abatacept for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2009 (4): CD007277. October 2009. doi:10.1002/14651858.CD007277.pub2. PMID 19821401.
- ↑ "Adalimumab for treating rheumatoid arthritis". The Cochrane Database of Systematic Reviews (3): CD005113. July 2005. doi:10.1002/14651858.CD005113.pub2. PMID 16034967.
- ↑ "Biologics for rheumatoid arthritis: an overview of Cochrane reviews". The Cochrane Database of Systematic Reviews 128 (4): CD007848. October 2009. doi:10.1002/14651858.CD007848.pub2. PMID 19821440.
- ↑ 130.0 130.1 "Biologic interventions for fatigue in rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2016 (6): CD008334. June 2016. doi:10.1002/14651858.cd008334.pub2. PMID 27271314.
- ↑ "Cyclosporine for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 1998 (2): CD001083. 2000. doi:10.1002/14651858.CD001083. PMID 10796412.
- ↑ "Moderate-term, low-dose corticosteroids for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2010 (2): CD001158. 1998-07-27. doi:10.1002/14651858.cd001158. PMID 10796420.
- ↑ "Effects of glucocorticoids on radiological progression in rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2010 (1): CD006356. January 2007. doi:10.1002/14651858.cd006356. PMID 17253590.
- ↑ "Intra-articular steroids and splints/rest for children with juvenile idiopathic arthritis and adults with rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2008 (1): CD002824. January 2006. doi:10.1002/14651858.cd002824.pub2. PMID 16437446.
- ↑ 135.0 135.1 "Combination therapy for pain management in inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis)". The Cochrane Database of Systematic Reviews (10): CD008886. October 2011. doi:10.1002/14651858.cd008886.pub2. PMID 21975788.
- ↑ "Effect of nonsteroidal antiinflammatory drugs on the C-reactive protein level in rheumatoid arthritis: a meta-analysis of randomized controlled trials". Arthritis and Rheumatism 64 (11): 3511–3521. November 2012. doi:10.1002/art.34644. PMID 22833186.
- ↑ "Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity". The Cochrane Database of Systematic Reviews 1 (2): CD008951. January 2012. doi:10.1002/14651858.CD008951.pub2. PMID 22258995.
- ↑ "Celecoxib: a review of its use for symptomatic relief in the treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis". Drugs 71 (18): 2457–2489. December 2011. doi:10.2165/11208240-000000000-00000. PMID 22141388.
- ↑ "Pain management for rheumatoid arthritis and cardiovascular or renal comorbidity". The Cochrane Database of Systematic Reviews (10): CD008952. October 2011. doi:10.1002/14651858.CD008952.pub2. PMID 21975789.
- ↑ "Rofecoxib for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2010 (1): CD003685. January 2005. doi:10.1002/14651858.cd003685.pub2. PMID 15674912.
- ↑ "Safety of non-steroidal anti-inflammatory drugs, including aspirin and paracetamol (acetaminophen) in people receiving methotrexate for inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis)". The Cochrane Database of Systematic Reviews (11): CD008872. November 2011. doi:10.1002/14651858.CD008872.pub2. PMID 22071858.
- ↑ "Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation". Health Technology Assessment 12 (11): 1–278, iii. April 2008. doi:10.3310/hta12110. PMID 18405470.
- ↑ "Celecoxib for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 6 (6): CD012095. June 2017. doi:10.1002/14651858.CD012095.pub2. PMID 28597983.
- ↑ 144.0 144.1 "Paracetamol versus nonsteroidal anti-inflammatory drugs for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2010 (1): CD003789. 2004-01-26. doi:10.1002/14651858.cd003789.pub2. PMID 14974037.
- ↑ 145.0 145.1 "Neuromodulators for pain management in rheumatoid arthritis". The Cochrane Database of Systematic Reviews 1 (1): CD008921. January 2012. doi:10.1002/14651858.CD008921.pub2. PMID 22258992.
- ↑ "Opioid therapy for treating rheumatoid arthritis pain". The Cochrane Database of Systematic Reviews (11): CD003113. November 2011. doi:10.1002/14651858.cd003113.pub3. PMID 22071805.
- ↑ "Surgical interventions for the rheumatoid shoulder". The Cochrane Database of Systematic Reviews (1): CD006188. January 2010. doi:10.1002/14651858.cd006188.pub2. PMID 20091587.
- ↑ 148.0 148.1 "Physiotherapy in rheumatoid arthritis". MedGenMed 6 (2): 3. May 2004. PMID 15266230.
- ↑ 149.0 149.1 "The effectiveness of home hand exercise programmes in rheumatoid arthritis: a systematic review". British Medical Bulletin 119 (1): 49–62. September 2016. doi:10.1093/bmb/ldw024. PMID 27365455.
- ↑ 150.0 150.1 "The practice of physical activity and cryotherapy in rheumatoid arthritis: systematic review". European Journal of Physical and Rehabilitation Medicine 53 (5): 775–787. October 2017. doi:10.23736/s1973-9087.16.04534-2. PMID 27996221.
- ↑ "Compression gloves for patients with hand arthritis (C-GLOVES): A feasibility study" (in en). Hand Therapy 26 (1): 26–37. 1 March 2021. doi:10.1177/1758998320986829. ISSN 1758-9983. PMID 37905193.
- ↑ "The effects of compression gloves on hand symptoms and hand function in rheumatoid arthritis and hand osteoarthritis: a systematic review". Clinical Rehabilitation 30 (3): 213–224. March 2016. doi:10.1177/0269215515578296. PMID 25802424. http://usir.salford.ac.uk/id/eprint/34121/1/SR%20compression%20gloves%20in%20RA%20HOA%20%20Clin%20Rehab%202015%20FINAL%20word%20version.pdf.
- ↑ "Rheumatoid Arthritis and Complementary Health Approaches". National Center for Complementary and Integrative Health. January 2006. http://nccih.nih.gov/health/RA/getthefacts.htm.
- ↑ "Evidence for the efficacy of complementary and alternative medicines in the management of rheumatoid arthritis: a systematic review". Rheumatology 50 (9): 1672–1683. September 2011. doi:10.1093/rheumatology/ker119. PMID 21652584.
- ↑ 155.0 155.1 "Complementary and alternative medicine use in rheumatoid arthritis: proposed mechanism of action and efficacy of commonly used modalities". Rheumatology International 30 (5): 571–586. March 2010. doi:10.1007/s00296-009-1206-y. PMID 19876631.
- ↑ "Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis". The Cochrane Database of Systematic Reviews 4 (4): CD002049. October 2005. doi:10.1002/14651858.CD002049.pub2. PMID 16235295.
- ↑ "Tai Chi for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 9 (9): CD004849. September 2019. doi:10.1002/14651858.CD004849.pub2. PMID 31553478.
- ↑ "Tai chi for rheumatoid arthritis: systematic review". Rheumatology 46 (11): 1648–1651. November 2007. doi:10.1093/rheumatology/kem151. PMID 17634188.
- ↑ "Acupuncture for rheumatoid arthritis: a systematic review". Rheumatology 47 (12): 1747–1753. December 2008. doi:10.1093/rheumatology/ken330. PMID 18710899.
- ↑ "A systematic review of evidence for the effectiveness of practitioner-based complementary and alternative therapies in the management of rheumatic diseases: rheumatoid arthritis". Rheumatology 51 (9): 1707–1713. September 2012. doi:10.1093/rheumatology/kes133. PMID 22661556.
- ↑ "Electrical stimulation for the treatment of rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2010 (2): CD003687. 2002. doi:10.1002/14651858.CD003687. PMID 12076504.
- ↑ "Penicillamine for treating rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2011 (4): CD001460. 2000-10-23. doi:10.1002/14651858.cd001460. PMID 11034719.
- ↑ 163.0 163.1 "Therapeutic ultrasound for the treatment of rheumatoid arthritis". The Cochrane Database of Systematic Reviews (3): CD003787. 2002-07-22. doi:10.1002/14651858.cd003787. PMID 12137714.
- ↑ 164.0 164.1 "Pain: continued uncertainty of TENS' effectiveness for pain relief". Nature Reviews. Rheumatology 6 (6): 314–316. June 2010. doi:10.1002/14651858.cd004377. PMID 20520646.
- ↑ 165.0 165.1 "Assistive technology for rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2009 (4): CD006729. October 2009. doi:10.1002/14651858.cd006729.pub2. PMID 19821383.
- ↑ "Balance training (proprioceptive training) for patients with rheumatoid arthritis". The Cochrane Database of Systematic Reviews (5): CD007648. May 2010. doi:10.1002/14651858.cd007648.pub2. PMID 20464755.
- ↑ "The role of nutrition and diet in rheumatoid arthritis". The Proceedings of the Nutrition Society 57 (2): 231–234. May 1998. doi:10.1079/pns19980036. PMID 9656325.
- ↑ "Is There a Role for Diet in the Therapy of Rheumatoid Arthritis?". Current Rheumatology Reports 18 (5): 23. May 2016. doi:10.1007/s11926-016-0575-y. PMID 27032786.
- ↑ "Herbal medicines for the treatment of rheumatoid arthritis: a systematic review". Rheumatology (National Institute for Health and Care Research) 42 (5): 652–659. May 2003. doi:10.1093/rheumatology/keg183. PMID 12709541. http://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=12003000980. Retrieved March 23, 2013.
- ↑ 170.0 170.1 "Marine Oil Supplements for Arthritis Pain: A Systematic Review and Meta-Analysis of Randomized Trials". Nutrients 9 (1): 42. January 2017. doi:10.3390/nu9010042. PMID 28067815.
- ↑ 171.0 171.1 "Effect of Marine-Derived n-3 Polyunsaturated Fatty Acids on Major Eicosanoids: A Systematic Review and Meta-Analysis from 18 Randomized Controlled Trials". PLOS ONE 11 (1): e0147351. 2016. doi:10.1371/journal.pone.0147351. PMID 26808318. Bibcode: 2016PLoSO..1147351J.
- ↑ 172.0 172.1 "Fish consumption and risk of rheumatoid arthritis: a dose-response meta-analysis". Arthritis Research & Therapy 16 (5): 446. September 2014. doi:10.1186/s13075-014-0446-8. PMID 25267142.
- ↑ 173.0 173.1 "Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis". Archives of Medical Research 43 (5): 356–362. July 2012. doi:10.1016/j.arcmed.2012.06.011. PMID 22835600.
- ↑ 174.0 174.1 "Herbal Remedies, Supplements and Acupuncture for Arthritis". American College of Rheumatology. http://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Herbal_Remedies,_Supplements_and_Acupuncture_for_Arthritis/.
- ↑ "Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2005 (3): CD000189. 2005-01-24. doi:10.1002/14651858.cd000189.pub2. PMID 15266426.
- ↑ "Vaccinations for rheumatoid arthritis". Current Rheumatology Reports 16 (8): 431. August 2014. doi:10.1007/s11926-014-0431-x. PMID 24925587.
- ↑ "Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014-15 influenza season". MMWR. Morbidity and Mortality Weekly Report 63 (32): 691–697. August 2014. PMID 25121712.
- ↑ "Influenza vaccination coverage among health care personnel--United States, 2013-14 influenza season". MMWR. Morbidity and Mortality Weekly Report 63 (37): 805–811. September 2014. PMID 25233281.
- ↑ "Update on recommendations for use of herpes zoster vaccine". MMWR. Morbidity and Mortality Weekly Report 63 (33): 729–731. August 2014. PMID 25144544.
- ↑ "WHO Disease and injury country estimates". 2009. https://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html.
- ↑ The New Harvard Guide to Women's Health. Cambridge, MA: Harvard University Press. 2004.
- ↑ Arthritis and You. Lanham, MD: Rowman & Littlefield Publishers, Inc.. 2013. pp. 138. ISBN 978-1-4422-1901-4. https://archive.org/details/arthritisyoucomp0000alin/page/138.
- ↑ "Impending radiographic erosive progression over the following year in a cohort of consecutive patients with inflammatory polyarthritis: prediction by serum biomarkers". RMD Open 6 (1): e001191. April 2020. doi:10.1136/rmdopen-2020-001191. PMID 32371434.
- ↑ "Serum levels of 14-3-3η protein supplement C-reactive protein and rheumatoid arthritis-associated antibodies to predict clinical and radiographic outcomes in a prospective cohort of patients with recent-onset inflammatory polyarthritis". Arthritis Research & Therapy 18 (37): 37. Feb 2016. doi:10.1186/s13075-016-0935-z. PMID 26832367.
- ↑ 185.0 185.1 Donahue, Katrina E.; Gartlehner, Gerald; Schulman, Elizabeth R.; Jonas, Beth; Coker-Schwimmer, Emmanuel; Patel, Sheila V.; Weber, Rachel Palmieri; Lohr, Kathleen N. et al. (2018). Drug Therapy for Early Rheumatoid Arthritis: A Systematic Review Update. AHRQ Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US). http://www.ncbi.nlm.nih.gov/books/NBK524950/.
- ↑ Kitas, George (4 April 2006) Why is life span shortened by Rheumatoid Arthritis? National Rheumatoid Arthritis Society
- ↑ Rheumatoid Arthritis Patients Have Double the Risk of Heart Failure. mayoclinic.org (3 February 2005).
- ↑ "Cardiac disease in rheumatoid arthritis". Johns Hopkins University. 2002. http://www.hopkins-arthritis.org/news-archive/2002/cardiac.html.
- ↑ "The effect of pharmacological therapy on the cardiovascular system of patients with systemic rheumatic diseases". Autoimmunity Reviews 9 (12): 835–839. October 2010. doi:10.1016/j.autrev.2010.07.018. PMID 20678592.
- ↑ "Biologics, cardiovascular effects and cancer". BMC Medicine 12 (1): 48. March 2014. doi:10.1186/1741-7015-12-48. PMID 24642038.
- ↑ "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet 380 (9859): 2095–2128. December 2012. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604. PMC 10790329. https://zenodo.org/record/2557786.
- ↑ "Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review". Seminars in Arthritis and Rheumatism 36 (3): 182–188. December 2006. doi:10.1016/j.semarthrit.2006.08.006. PMID 17045630.
- ↑ "Periodontal disease and periodontal bacteria as triggers for rheumatoid arthritis". Best Practice & Research. Clinical Rheumatology 31 (1): 19–30. February 2017. doi:10.1016/j.berh.2017.08.001. PMID 29221594. http://eprints.whiterose.ac.uk/119931/1/Cheng_et_al_PDRA%20review_final.pdf.
- ↑ The Nature and Treatment of Gout and Rheumatic Gout. London: Walton and Maberly. 1859.
- ↑ "Rubens and the question of antiquity of rheumatoid arthritis". JAMA 245 (5): 483–486. February 1981. doi:10.1001/jama.245.5.483. PMID 7005475.
- ↑ "Did RA travel from New World to Old? The Rubens connection". Medscape. 14 June 2005. http://www.medscape.com/viewarticle/538251.
- ↑ "Rheumatoid arthritis-like deformities in an early 16th-century painting of the Flemish-Dutch school". JAMA 268 (2): 249–251. July 1992. doi:10.1001/jama.268.2.249. PMID 1608144.
- ↑ "History of the treatment of rheumatoid arthritis". British Medical Journal 1 (6012): 763–765. March 1976. doi:10.1136/bmj.1.6012.763. PMID 177148.
- ↑ "A Possible Link Between Rheumatoid Arthritis and Periodontitis: A Systematic Review and Meta-analysis". The International Journal of Periodontics & Restorative Dentistry 37 (1): 79–86. 2017-02-01. doi:10.11607/prd.2656. PMID 27977821.
- ↑ "Vitamin D deficiency and risk for rheumatic diseases: an update". Current Opinion in Rheumatology 25 (2): 184–191. March 2013. doi:10.1097/BOR.0b013e32835cfc16. PMID 23370372.
- ↑ "Vitamin D, immunoregulation, and rheumatoid arthritis". Journal of Clinical Rheumatology 17 (2): 102–107. March 2011. doi:10.1097/RHU.0b013e31820edd18. PMID 21364350.
- ↑ "Vitamin D and inflammation". Joint, Bone, Spine 77 (6): 552–557. December 2010. doi:10.1016/j.jbspin.2010.09.018. PMID 21067953.
- ↑ "Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: a meta-analysis". Clinical and Experimental Rheumatology 34 (5): 827–833. 2016. PMID 27049238. http://www.clinexprheumatol.org/article.asp?a=10111.
- ↑ "Intestinal barrier dysfunction plays an integral role in arthritis pathology and can be targeted to ameliorate disease". Med 2 (7): 864–883.e9. July 2021. doi:10.1016/j.medj.2021.04.013. PMID 34296202.
External links
| Classification | |
|---|---|
| External resources |
- Rheumatoid arthritis at Curlie
- "Rheumatoid Arthritis". MedlinePlus. U.S. National Library of Medicine. https://medlineplus.gov/rheumatoidarthritis.html.
 |