Chemistry:Pacritinib
 | |
| Clinical data | |
|---|---|
| Trade names | Vonjo |
| Other names | SB1518 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
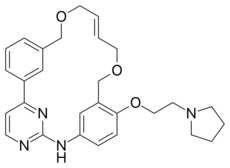
| Formula | C28H32N4O3 |
| Molar mass | 472.589 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pacritinib, sold under the brand name Vonjo, is an anti-cancer medication used to treat myelofibrosis.[1][2] It is a macrocyclic protein kinase inhibitor. It mainly inhibits Janus kinase 2 (JAK2) and Fms-like tyrosine kinase 3\CD135 (FLT3).
Common side effects include diarrhea, low platelet counts, nausea, anemia, and swelling in legs.[2]
Medical uses
Pacritinib in indicated to treat adults who have a rare form of a bone marrow disorder known as intermediate or high-risk primary or secondary myelofibrosis and who have platelet (blood clotting cells) levels below 50,000/µL.[1][2]
History
The effectiveness and safety of pacritinib were demonstrated in a study that included 63 participants with intermediate or high-risk primary or secondary myelofibrosis and low platelets who received pacritinib 200 mg twice daily or standard treatment.[2] Effectiveness was determined based upon the proportion of participants who had a 35% or greater spleen volume reduction from baseline to week 24.[2] Nine participants (29%) in the pacritinib treatment group had a 35% or greater spleen volume reduction, compared to one participant (3%) in the standard treatment group.[2]
The U.S. Food and Drug Administration (FDA) granted the application for pacritinib priority review, fast track, and orphan drug designations.[2]
Society and culture
Names
Pacritinib is the International nonproprietary name (INN).[3][4]
References
- ↑ 1.0 1.1 1.2 "Vonjo- pacritinib capsule". 7 March 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4b5ab444-0e1a-4984-99db-76ad11a298ee.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "FDA approves drug for adults with rare form of bone marrow disorder". 1 March 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-adults-rare-form-bone-marrow-disorder.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "International nonproprietary names for pharmaceutical substances (INN). proposed INN: list 104". WHO Drug Information 24 (4): 386. 2010. https://www.who.int/medicines/publications/druginformation/innlists/PL104.pdf.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 66". WHO Drug Information 25 (3). 2011.
External links
- "Pacritinib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/pacritinib.
 |

