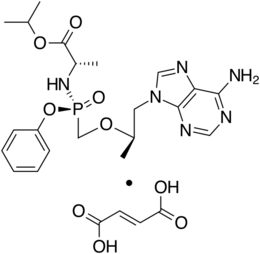
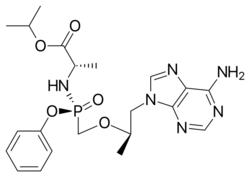
Chemistry:Tenofovir alafenamide
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌtəˈnoʊfəvɪər ˌæləˈfɛnəmaɪd/ |
| Trade names | Vemlidy Genvoya (with elvitegravir, cobicistat and emtricitabine) Odefsey (with emtricitabine and rilpivirine) Descovy (with emtricitabine) Symtuza (with darunavir, cobicistat, and emtricitabine) |
| Other names | GS-7340 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ~80%[5] |
| Elimination half-life | 0.51 hour |
| Excretion | Feces (31.7%), urine (<1%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H29N6O5P |
| Molar mass | 476.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tenofovir alafenamide, sold under the brand name Vemlidy, is an antiviral medication used against hepatitis B and HIV. It is used for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease[7] and is given in combination with other medications for the prevention and treatment of HIV. It is taken by mouth.[5]
Tenofovir alafenamide is a nucleotide reverse transcriptase inhibitor and is a prodrug of tenofovir. It was developed by Gilead Sciences based on the protide technology of Chris McGuigan and is applied in the form of tenofovir alafenamide fumarate (TAF). Closely related to the commonly used reverse-transcriptase inhibitor tenofovir disoproxil fumarate (TDF), TAF has greater antiviral activity and better distribution into lymphoid tissues than that agent.[8][9] It was approved for use in the US against HIV in 2015[10] and against hepatitis B in 2016.[11] It is available as a generic medication.[12]
Fixed-dose combinations containing tenofovir alafenamide
- Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (Genvoya)[13] — approved both in the United States and in the European Union in November 2015[14][15][16] (compare elvitegravir/cobicistat/emtricitabine/tenofovir; (Stribild)[17][18][19][20])
- Emtricitabine/rilpivirine/tenofovir alafenamide (Odefsey)[21] — approved in the United States in March 2016, and in the European Union in June 2016[22][23] (compare Emtricitabine/rilpivirine/tenofovir; (Complera)[24][25][26])
- Emtricitabine/tenofovir alafenamide (Descovy)[27] — approved in the United States in April 2016 (compare emtricitabine/tenofovir; (Truvada)). In October 2019, Descovy was approved in the United States for HIV-1 pre-exposure prophylaxis (PrEP).[28][29]
- Bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy)[30] — approved in the United States in February 2018.
- Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (Symtuza)[31] — approved in the European Union in September 2017, in the United States in July 2018, and in Australia in November 2019.[32][33][34][35]
- Dolutegravir/emtricitabine/tenofovir alafenamide.[36]
- Dolutegravir/lamivudine/tenofovir alafenamide.[37][38]
Research
Gilead announced a Phase III clinical trial evaluating a single-tablet regimen combining tenofovir alafenamide with cobicistat, emtricitabine and elvitegravir[39] and developed a coformulation of the drug with cobicistat, emtricitabine and the protease inhibitor darunavir.[40][41][42] In a 48-week study comparing elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil (Stribild) to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (Genvoya), the results showed the newer drug's effects to be non-inferior to the established agent, but at much lower dosages and with lower incidence of adverse side effects such as impaired kidney function.[43][44][45] The FDA approved the TAF-based treatment regimen for treatment of HIV-1 in November 2015.[10] Genvoya is the first TAF-based regimen to receive approval.[10]
References
- ↑ "Tenofovir alafenamide (Vemlidy) Use During Pregnancy". 26 December 2018. https://www.drugs.com/pregnancy/tenofovir-alafenamide.html.
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2016". 21 June 2022. https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2016.
- ↑ "Product Monograph: Vemlidy (tenofovir alafenamide) tablets". Government of Canada: The Drug and Health Product Register. 20 August 2020. https://pdf.hres.ca/dpd_pm/00058101.PDF.
- ↑ "Vemlidy 25 mg film coated tablets - Summary of Product Characteristics (SmPC)". 8 September 2020. https://www.medicines.org.uk/emc/product/2314.
- ↑ 5.0 5.1 5.2 "Vemlidy- tenofovir alafenamide tablet". DailyMed. U.S. National Library of Medicine. 11 February 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=72e6b33c-0351-4070-9172-eeaa186c01d2.
- ↑ "Vemlidy EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/vemlidy.
- ↑ "Tenofovir Alafenamide for the Treatment of Chronic Hepatitis B Monoinfection". Pharmacotherapy 38 (10): 1051–1057. October 2018. doi:10.1002/phar.2174. PMID 30120841.
- ↑ "Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood". Nucleosides Nucleotides Nucleic Acids 20 (4–7): 1091–8. 2001. doi:10.1081/NCN-100002496. PMID 11562963.
- ↑ "GS-7340 demonstrates greater declines in HIV-1 RNA than tenofovir disoproxil fumarate during 14 days of monotherapy in HIV-1 infected subjects.". 18th Conference on Retroviruses and Opportunistic Infections. March 2011. http://hivandhepatitis.com/2011_conference/croi2011/posters/diamond.pdf.
- ↑ 10.0 10.1 10.2 "U.S. Food and Drug Administration Approves Gilead's Single Tablet Regimen Genvoya (Elvitegravir, Cobicistat, Emtricitabine and Tenofovir Alafenamide) for Treatment of HIV-1 Infection" (Press release). Gilead. 5 November 2015. Archived from the original on 2015-11-08.
- ↑ "FDA Approves Vemlidy (tenofovir alafenamide) for Chronic Hepatitis B in Adults". 21 November 2016. https://www.hhs.gov/hepatitis/blog/2016/11/21/fda-approves-vemlidy-tenofovir-alafenamide-for-chronic-hepatitis-b-in-adults.html.
- ↑ "First Generic Drug Approvals 2023". 30 May 2023. https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals.
- ↑ "Genvoya- elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide tablet". DailyMed. U.S. National Library of Medicine. 11 February 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=34784acf-15ed-4715-b504-eb30430518e9.
- ↑ "Genvoya (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) fixed-dose combination tablet". 8 December 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207561Orig1s000TOC.cfm.
- ↑ "Summary Review: Genvoya". US Food and Drug Administration. 6 August 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207561Orig1s000SumR.pdf.
- ↑ "Genvoya EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/genvoya.
- ↑ "Drug Approval Package: Stribild (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) Fixed Dose". 10 October 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203100Orig1s000TOC.cfm.
- ↑ "Summary Review: Stribild". US Food and Drug Administration. 19 October 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203100Orig1s000SumR.pdf.
- ↑ "Stribild- elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate tablet, film coated". DailyMed. U.S. National Library of Medicine. 28 January 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=74ae2a93-b267-444c-8f0f-a2a6522260ee.
- ↑ "Stribild EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/stribild.
- ↑ "Odefsey- emtricitabine, rilpivirine hydrochloride, and tenofovir alafenamide tablet". DailyMed. U.S. National Library of Medicine. 6 December 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ea3b9ec8-e04a-412c-8b3f-e5cbc7e641d5.
- ↑ "Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide) Tablets". 29 November 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208351Orig1s000TOC.cfm.
- ↑ "Odefsey EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/odefsey.
- ↑ "Drug Approval Package: (emtricitabine/rilpivirine/tenofovir disoproxil fumarate) NDA #202123". 6 September 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202123_compleratm_toc.cfm.
- ↑ "Summary Review: Complera". US Food and Drug Administration. 19 July 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202123Orig1s000SumR.pdf.
- ↑ "Complera- emtricitabine, rilpivirine hydrochloride, and tenofovir disoproxil fumarate tablet, film coated". DailyMed. U.S. National Library of Medicine. 9 December 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d637cfab-f1e8-4eb3-a1b3-f85ca3bec612.
- ↑ "Descovy- emtricitabine and tenofovir alafenamide tablet". DailyMed. U.S. National Library of Medicine. 13 January 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=06f66e98-e6ee-4538-9506-6c1282cc14c1.
- ↑ "FDA approves second drug to prevent HIV infection as part of ongoing efforts to end the HIV epidemic". 3 October 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-second-drug-prevent-hiv-infection-part-ongoing-efforts-end-hiv-epidemic.
- ↑ "F.D.A. Approves New H.I.V.-Prevention Drug, but Not for Everyone". 4 October 2019. https://www.nytimes.com/2019/10/04/health/fda-descovy-truvada-hiv.html.
- ↑ "Biktarvy- bictegravir sodium, emtricitabine, and tenofovir alafenamide fumarate tablet". DailyMed. U.S. National Library of Medicine. 8 August 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=664cb8f0-1f65-441b-b0d9-ba3d798be309.
- ↑ "Symtuza- darunavir, cobicistat, emtricitabine, and tenofovir alafenamide tablet, film coated". DailyMed. U.S. National Library of Medicine. 6 March 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=85a17d00-6b7c-41ea-a6b3-5ad924820dab.
- ↑ "Drug Approval Package: Symtuza (darunavir, cobicistat, emtricitabine, and tenofovir alafenamide)". 11 December 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210455Orig1s000TOC.cfm.
- ↑ "Symtuza EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/symtuza.
- ↑ "TGA eBS - Product and Consumer Medicine Information Licence". https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2019-PI-02280-1.
- ↑ "Symtuza 800/150/200/10 Tablets". 15 July 2021. https://www.nps.org.au/medicine-finder/symtuza-800-150-200-10-tablets#full-pi.
- ↑ "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=213681.
- ↑ "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=212527.
- ↑ "Tentative Approval: Dolutegravir, Lamivudine, and Tenofovir Alafenamide Tablets". US Food and Drug Administration. 30 March 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/212527Orig1s000TAltr.pdf.
- ↑ "Gilead Initiates Phase 3 Clinical Program for Tenofovir Alafenamide, a Novel Low-Dose Prodrug for the Treatment of HIV" (Press release). Gilead. 24 January 2013. Archived from the original on 2019-10-11.
- ↑ "Gilead Sciences Finalizes Agreement with Tibotec Pharmaceuticals to Develop and Commercialize a Single-Tablet Regimen of Prezista with Emtriva, GS 7340 and Cobicistat". Gilead Sciences (Press release). 15 November 2011. Archived from the original on 11 October 2019. Retrieved 10 October 2019.
- ↑ "GS-7340 Packs Greater HIV Punch, Potentially Better Safety, Versus Viread". AIDSmeds.com. 15 March 2012. http://www.aidsmeds.com/articles/hiv_7340_tenofovir_1667_22087.shtml.
- ↑ "Pharmacokinetics of a Novel EVG/COBI/FTC/GS-7340 Single Tablet Regimen". 13th International Workshop on Clinical Pharmacology of HIV Therapy.. Barcelona, Spain. April 2012. http://www.natap.org/2012/pharm/Pharm_24.htm.
- ↑ Once-Daily Tenofovir Prodrug Combo Pill as Effective as Stribild . AIDSmeds.com 1 Nov 2012.
- ↑ CROI 2013: New Pro-drug Tenofovir Alafenamide Appears Equally Effective but Better Tolerated . Highleyman, Liz. HIVandHepatitis.com. 6 March 2013.
- ↑ "Tenefovir Alafenamide Fumarate (TAF) Sign-On Letter to Gilead". Treatment Action Group. 13 June 2013. http://www.treatmentactiongroup.org/hiv/taf-letter.
External links
- "Tenofovir alafenamide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tenofovir%20alafenamide.
- "Tenofovir alafenamide fumarate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tenofovir%20alafenamide%20fumarate.
 |