Chemistry:Oganesson
Oganesson is a synthetic chemical element; it has symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint team of Russian and American scientists. In December 2015, it was recognized as one of four new elements by the Joint Working Party of the international scientific bodies IUPAC and IUPAP. It was formally named on 28 November 2016.[1][2] The name honors the nuclear physicist Yuri Oganessian, who played a leading role in the discovery of the heaviest elements in the periodic table. It is one of only two elements named after a person who was alive at the time of naming, the other being seaborgium, and the only element whose eponym is alive (As of 2024).[3][lower-alpha 1]
Oganesson has the highest atomic number and highest atomic mass of all known elements (As of 2024). On the periodic table of the elements it is a p-block element, a member of group 18 and the last member of period 7. Its only known isotope, oganesson-294, is highly radioactive, with a half-life of 0.7 ms and, (As of 2020) only five atoms have been successfully produced.[5] This has so far prevented any experimental studies of its chemistry. Because of relativistic effects, theoretical studies predict that it would be a solid at room temperature, and significantly reactive,[5][6] unlike the other members of group 18 (the noble gases).
Introduction
History
Early speculation
The possibility of a seventh noble gas, after helium, neon, argon, krypton, xenon, and radon, was considered almost as soon as the noble gas group was discovered. Danish chemist Hans Peter Jørgen Julius Thomsen predicted in April 1895, the year after the discovery of argon, that there was a whole series of chemically inert gases similar to argon that would bridge the halogen and alkali metal groups: he expected that the seventh of this series would end a 32-element period which contained thorium and uranium and have an atomic weight of 292, close to the 294 now known for the first and only confirmed isotope of oganesson.[7] Danish physicist Niels Bohr noted in 1922 that this seventh noble gas should have atomic number 118 and predicted its electronic structure as 2, 8, 18, 32, 32, 18, 8, matching modern predictions.[8] Following this, German chemist Aristid von Grosse wrote an article in 1965 predicting the likely properties of element 118.[9] It was 107 years from Thomsen's prediction before oganesson was successfully synthesized, although its chemical properties have not been investigated to determine if it behaves as the heavier congener of radon.[10] In a 1975 article, American chemist Kenneth Pitzer suggested that element 118 should be a gas or volatile liquid due to relativistic effects.[11]
Unconfirmed discovery claims
In late 1998, Polish physicist Robert Smolańczuk published calculations on the fusion of atomic nuclei towards the synthesis of superheavy atoms, including oganesson.[12] His calculations suggested that it might be possible to make element 118 by fusing lead with krypton under carefully controlled conditions, and that the fusion probability (cross section) of that reaction would be close to the lead–chromium reaction that had produced element 106, seaborgium. This contradicted predictions that the cross sections for reactions with lead or bismuth targets would go down exponentially as the atomic number of the resulting elements increased.[12]
In 1999, researchers at Lawrence Berkeley National Laboratory made use of these predictions and announced the discovery of elements 118 and 116, in a paper published in Physical Review Letters,[13] and very soon after the results were reported in Science.[14] The researchers reported that they had performed the reaction
In 2001, they published a retraction after researchers at other laboratories were unable to duplicate the results and the Berkeley lab could not duplicate them either.[15] In June 2002, the director of the lab announced that the original claim of the discovery of these two elements had been based on data fabricated by principal author Victor Ninov.[16][17] Newer experimental results and theoretical predictions have confirmed the exponential decrease in cross sections with lead and bismuth targets as the atomic number of the resulting nuclide increases.[18]
Discovery reports
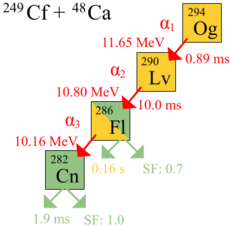
The first genuine decay of atoms of oganesson was observed in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, by a joint team of Russian and American scientists. Headed by Yuri Oganessian, a Russian nuclear physicist of Armenian ethnicity, the team included American scientists from the Lawrence Livermore National Laboratory in California.[20] The discovery was not announced immediately, because the decay energy of 294Og matched that of 212mPo, a common impurity produced in fusion reactions aimed at producing superheavy elements, and thus announcement was delayed until after a 2005 confirmatory experiment aimed at producing more oganesson atoms.[21] The 2005 experiment used a different beam energy (251 MeV instead of 245 MeV) and target thickness (0.34 mg/cm2 instead of 0.23 mg/cm2).[19] On 9 October 2006, the researchers announced[19] that they had indirectly detected a total of three (possibly four) nuclei of oganesson-294 (one or two in 2002[22] and two more in 2005) produced via collisions of californium-249 atoms and calcium-48 ions.[23][24][25][26][27]
In 2011, IUPAC evaluated the 2006 results of the Dubna–Livermore collaboration and concluded: "The three events reported for the Z = 118 isotope have very good internal redundancy but with no anchor to known nuclei do not satisfy the criteria for discovery".[28]
Because of the very small fusion reaction probability (the fusion cross section is ~0.3–0.6 pb or (3–6)×10−41 m2) the experiment took four months and involved a beam dose of 2.5×1019 calcium ions that had to be shot at the californium target to produce the first recorded event believed to be the synthesis of oganesson.[29] Nevertheless, researchers were highly confident that the results were not a false positive, since the chance that the detections were random events was estimated to be less than one part in 100000.[30]
In the experiments, the alpha-decay of three atoms of oganesson was observed. A fourth decay by direct spontaneous fission was also proposed. A half-life of 0.89 ms was calculated: 294Og decays into 290Lv by alpha decay. Since there were only three nuclei, the half-life derived from observed lifetimes has a large uncertainty: 0.89+1.07
−0.31 ms.[19]
- 294118Og → 290116Lv + 42He
The identification of the 294Og nuclei was verified by separately creating the putative daughter nucleus 290Lv directly by means of a bombardment of 245Cm with 48Ca ions,
- 24596Cm + 4820Ca → 290116Lv + 3 Neutron,
and checking that the 290Lv decay matched the decay chain of the 294Og nuclei.[19] The daughter nucleus 290Lv is very unstable, decaying with a lifetime of 14 milliseconds into 286Fl, which may experience either spontaneous fission or alpha decay into 282Cn, which will undergo spontaneous fission.[19]
Confirmation
In December 2015, the Joint Working Party of international scientific bodies International Union of Pure and Applied Chemistry (IUPAC) and International Union of Pure and Applied Physics (IUPAP) recognized the element's discovery and assigned the priority of the discovery to the Dubna–Livermore collaboration.[31] This was on account of two 2009 and 2010 confirmations of the properties of the granddaughter of 294Og, 286Fl, at the Lawrence Berkeley National Laboratory, as well as the observation of another consistent decay chain of 294Og by the Dubna group in 2012. The goal of that experiment had been the synthesis of 294Ts via the reaction 249Bk(48Ca,3n), but the short half-life of 249Bk resulted in a significant quantity of the target having decayed to 249Cf, resulting in the synthesis of oganesson instead of tennessine.[32]
From 1 October 2015 to 6 April 2016, the Dubna team performed a similar experiment with 48Ca projectiles aimed at a mixed-isotope californium target containing 249Cf, 250Cf, and 251Cf, with the aim of producing the heavier oganesson isotopes 295Og and 296Og. Two beam energies at 252 MeV and 258 MeV were used. Only one atom was seen at the lower beam energy, whose decay chain fitted the previously known one of 294Og (terminating with spontaneous fission of 286Fl), and none were seen at the higher beam energy. The experiment was then halted, as the glue from the sector frames covered the target and blocked evaporation residues from escaping to the detectors.[33] The production of 293Og and its daughter 289Lv, as well as the even heavier isotope 297Og, is also possible using this reaction. The isotopes 295Og and 296Og may also be produced in the fusion of 248Cm with 50Ti projectiles.[33][34][35] A search beginning in summer 2016 at RIKEN for 295Og in the 3n channel of this reaction was unsuccessful, though the study is planned to resume; a detailed analysis and cross section limit were not provided. These heavier and likely more stable isotopes may be useful in probing the chemistry of oganesson.[36][37]
Naming
Using Mendeleev's nomenclature for unnamed and undiscovered elements, oganesson is sometimes known as eka-radon (until the 1960s as eka-emanation, emanation being the old name for radon).[9] In 1979, IUPAC assigned the systematic placeholder name ununoctium to the undiscovered element, with the corresponding symbol of Uuo,[38] and recommended that it be used until after confirmed discovery of the element.[39] Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations were mostly ignored among scientists in the field, who called it "element 118", with the symbol of E118, (118), or even simply 118.[40]
Before the retraction in 2001, the researchers from Berkeley had intended to name the element ghiorsium (Gh), after Albert Ghiorso (a leading member of the research team).[41]
The Russian discoverers reported their synthesis in 2006. According to IUPAC recommendations, the discoverers of a new element have the right to suggest a name.[42] In 2007, the head of the Russian institute stated the team were considering two names for the new element: flyorium, in honor of Georgy Flyorov, the founder of the research laboratory in Dubna; and moskovium, in recognition of the Moscow Oblast where Dubna is located.[43] He also stated that although the element was discovered as an American collaboration, who provided the californium target, the element should rightly be named in honor of Russia since the Flyorov Laboratory of Nuclear Reactions at JINR was the only facility in the world which could achieve this result.[44] These names were later suggested for element 114 (flerovium) and element 116 (moscovium).[45] Flerovium became the name of element 114; the final name proposed for element 116 was instead livermorium,[46] with moscovium later being proposed and accepted for element 115 instead.[3]
Traditionally, the names of all noble gases end in "-on", with the exception of helium, which was not known to be a noble gas when discovered. The IUPAC guidelines valid at the moment of the discovery approval however required all new elements be named with the ending "-ium", even if they turned out to be halogens (traditionally ending in "-ine") or noble gases (traditionally ending in "-on").[47] While the provisional name ununoctium followed this convention, a new IUPAC recommendation published in 2016 recommended using the "-on" ending for new group 18 elements, regardless of whether they turn out to have the chemical properties of a noble gas.[48]
The scientists involved in the discovery of element 118, as well as those of 117 and 115, held a conference call on 23 March 2016 to decide their names. Element 118 was the last to be decided upon; after Oganessian was asked to leave the call, the remaining scientists unanimously decided to have the element "oganesson" after him. Oganessian was a pioneer in superheavy element research for sixty years reaching back to the field's foundation: his team and his proposed techniques had led directly to the synthesis of elements 107 through 118. Mark Stoyer, a nuclear chemist at the LLNL, later recalled, "We had intended to propose that name from Livermore, and things kind of got proposed at the same time from multiple places. I don't know if we can claim that we actually proposed the name, but we had intended it."[49]
In internal discussions, IUPAC asked the JINR if they wanted the element to be spelled "oganeson" to match the Russian spelling more closely. Oganessian and the JINR refused this offer, citing the Soviet-era practice of transliterating names into the Latin alphabet under the rules of the French language ("Oganessian" is such a transliteration) and arguing that "oganesson" would be easier to link to the person.[50][lower-alpha 2] In June 2016, IUPAC announced that the discoverers planned to give the element the name oganesson (symbol: Og). The name became official on 28 November 2016.[3] In 2017, Oganessian commented on the naming:[51]
For me, it is an honour. The discovery of element 118 was by scientists at the Joint Institute for Nuclear Research in Russia and at the Lawrence Livermore National Laboratory in the US, and it was my colleagues who proposed the name oganesson. My children and grandchildren have been living in the US for decades, but my daughter wrote to me to say that she did not sleep the night she heard because she was crying.[51]
– Yuri Oganessian
The naming ceremony for moscovium, tennessine, and oganesson was held on 2 March 2017 at the Russian Academy of Sciences in Moscow.[52]
In a 2019 interview, when asked what it was like to see his name in the periodic table next to Einstein, Mendeleev, the Curies, and Rutherford, Oganessian responded:[50]
Not like much! You see, not like much. It is customary in science to name something new after its discoverer. It's just that there are few elements, and this happens rarely. But look at how many equations and theorems in mathematics are named after somebody. And in medicine? Alzheimer, Parkinson. There's nothing special about it.
Characteristics
Other than nuclear properties, no properties of oganesson or its compounds have been measured; this is due to its extremely limited and expensive production[53] and the fact that it decays very quickly. Thus only predictions are available.
Nuclear stability and isotopes
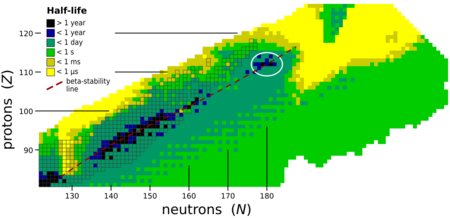
The stability of nuclei quickly decreases with the increase in atomic number after curium, element 96, whose most stable isotope, 247Cm, has a half-life four orders of magnitude longer than that of any subsequent element. All nuclides with an atomic number above 101 undergo radioactive decay with half-lives shorter than 30 hours. No elements with atomic numbers above 82 (after lead) have stable isotopes.[54] This is because of the ever-increasing Coulomb repulsion of protons, so that the strong nuclear force cannot hold the nucleus together against spontaneous fission for long. Calculations suggest that in the absence of other stabilizing factors, elements with more than 104 protons should not exist.[55] However, researchers in the 1960s suggested that the closed nuclear shells around 114 protons and 184 neutrons should counteract this instability, creating an island of stability in which nuclides could have half-lives reaching thousands or millions of years. While scientists have still not reached the island, the mere existence of the superheavy elements (including oganesson) confirms that this stabilizing effect is real, and in general the known superheavy nuclides become exponentially longer-lived as they approach the predicted location of the island.[56][57] Oganesson is radioactive, decaying via alpha decay and spontaneous fission,[58][59] with a half-life that appears to be less than a millisecond. Nonetheless, this is still longer than some predicted values.[60][61]
Calculations using a quantum-tunneling model predict the existence of several heavier isotopes of oganesson with alpha-decay half-lives close to 1 ms.[62][63]
Theoretical calculations done on the synthetic pathways for, and the half-life of, other isotopes have shown that some could be slightly more stable than the synthesized isotope 294Og, most likely 293Og, 295Og, 296Og, 297Og, 298Og, 300Og and 302Og (the last reaching the N = 184 shell closure).[60][64] Of these, 297Og might provide the best chances for obtaining longer-lived nuclei,[60][64] and thus might become the focus of future work with this element. Some isotopes with many more neutrons, such as some located around 313Og, could also provide longer-lived nuclei.[65]
In a quantum-tunneling model, the alpha decay half-life of 294Og was predicted to be 0.66+0.23
−0.18 ms[60] with the experimental Q-value published in 2004.[66] Calculation with theoretical Q-values from the macroscopic-microscopic model of Muntian–Hofman–Patyk–Sobiczewski gives somewhat lower but comparable results.[67]
Calculated atomic and physical properties
Oganesson is a member of group 18, the zero-valence elements. The members of this group are usually inert to most common chemical reactions (for example, combustion) because the outer valence shell is completely filled with eight electrons. This produces a stable, minimum energy configuration in which the outer electrons are tightly bound.[68] It is thought that similarly, oganesson has a closed outer valence shell in which its valence electrons are arranged in a 7s27p6 configuration.[6]
Consequently, some expect oganesson to have similar physical and chemical properties to other members of its group, most closely resembling the noble gas above it in the periodic table, radon.[69] Following the periodic trend, oganesson would be expected to be slightly more reactive than radon. However, theoretical calculations have shown that it could be significantly more reactive.[70] In addition to being far more reactive than radon, oganesson may be even more reactive than the elements flerovium and copernicium, which are heavier homologs of the more chemically active elements lead and mercury respectively.[6] The reason for the possible enhancement of the chemical activity of oganesson relative to radon is an energetic destabilization and a radial expansion of the last occupied 7p-subshell.[6] More precisely, considerable spin–orbit interactions between the 7p electrons and the inert 7s electrons effectively lead to a second valence shell closing at flerovium, and a significant decrease in stabilization of the closed shell of oganesson.[6] It has also been calculated that oganesson, unlike the other noble gases, binds an electron with release of energy, or in other words, it exhibits positive electron affinity,[71][72] due to the relativistically stabilized 8s energy level and the destabilized 7p3/2 level,[73] whereas copernicium and flerovium are predicted to have no electron affinity.[74][75] Nevertheless, quantum electrodynamic corrections have been shown to be quite significant in reducing this affinity by decreasing the binding in the anion Og− by 9%, thus confirming the importance of these corrections in superheavy elements.[71] 2022 calculations expect the electron affinity of oganesson to be 0.080(6) eV.[76]
Monte Carlo simulations of oganesson's molecular dynamics predict it has a melting point of 325±15 K and a boiling point of 450±10 K due to relativistic effects (if these effects are ignored, oganesson would melt at ≈220 K). Thus oganesson would probably be a solid rather than a gas under standard conditions, though still with a rather low melting point.[77][5]
Oganesson is expected to have an extremely broad polarizability, almost double that of radon.[6] Because of its tremendous polarizability, oganesson is expected to have an anomalously low first ionization energy of about 860 kJ/mol, similar to that of cadmium and less than those of iridium, platinum, and gold. This is significantly smaller than the values predicted for darmstadtium, roentgenium, and copernicium, although it is greater than that predicted for flerovium.[78] Its second ionization energy should be around 1560 kJ/mol.[76] Even the shell structure in the nucleus and electron cloud of oganesson is strongly impacted by relativistic effects: the valence and core electron subshells in oganesson are expected to be "smeared out" in a homogeneous Fermi gas of electrons, unlike those of the "less relativistic" radon and xenon (although there is some incipient delocalisation in radon), due to the very strong spin–orbit splitting of the 7p orbital in oganesson.[79] A similar effect for nucleons, particularly neutrons, is incipient in the closed-neutron-shell nucleus 302Og and is strongly in force at the hypothetical superheavy closed-shell nucleus 472164, with 164 protons and 308 neutrons.[79] Studies have also predicted that due to increasing electrostatic forces, oganesson may have a semibubble structure in proton density, having few protons at the center of its nucleus.[80][81] Moreover, spin–orbit effects may cause bulk oganesson to be a semiconductor, with a band gap of 1.5±0.6 eV predicted. All the lighter noble gases are insulators instead: for example, the band gap of bulk radon is expected to be 7.1±0.5 eV.[82]
Predicted compounds
The only confirmed isotope of oganesson, 294Og, has much too short a half-life to be chemically investigated experimentally. Therefore, no compounds of oganesson have been synthesized yet.[21] Nevertheless, calculations on theoretical compounds have been performed since 1964.[9] It is expected that if the ionization energy of the element is high enough, it will be difficult to oxidize and therefore, the most common oxidation state would be 0 (as for the noble gases);[83] nevertheless, this appears not to be the case.[10]
Calculations on the diatomic molecule Og2 showed a bonding interaction roughly equivalent to that calculated for Hg2, and a dissociation energy of 6 kJ/mol, roughly 4 times of that of Rn2.[6] Most strikingly, it was calculated to have a bond length shorter than in Rn2 by 0.16 Å, which would be indicative of a significant bonding interaction.[6] On the other hand, the compound OgH+ exhibits a dissociation energy (in other words proton affinity of oganesson) that is smaller than that of RnH+.[6]
The bonding between oganesson and hydrogen in OgH is predicted to be very weak and can be regarded as a pure van der Waals interaction rather than a true chemical bond.[84] On the other hand, with highly electronegative elements, oganesson seems to form more stable compounds than for example copernicium or flerovium.[84] The stable oxidation states +2 and +4 have been predicted to exist in the fluorides OgF2 and OgF4.[85] The +6 state would be less stable due to the strong binding of the 7p1/2 subshell.[10] This is a result of the same spin–orbit interactions that make oganesson unusually reactive. For example, it was shown that the reaction of oganesson with F2 to form the compound OgF2 would release an energy of 106 kcal/mol of which about 46 kcal/mol come from these interactions.[84] For comparison, the spin–orbit interaction for the similar molecule RnF2 is about 10 kcal/mol out of a formation energy of 49 kcal/mol.[84] The same interaction stabilizes the tetrahedral Td configuration for OgF4, as distinct from the square planar D4h one of XeF4, which RnF4 is also expected to have;[85] this is because OgF4 is expected to have two inert electron pairs (7s and 7p1/2). As such, OgF6 is expected to be unbound, continuing an expected trend in the destabilisation of the +6 oxidation state (RnF6 is likewise expected to be much less stable than XeF6).[86][87] The Og–F bond will most probably be ionic rather than covalent, rendering the oganesson fluorides non-volatile.[70][88] OgF2 is predicted to be partially ionic due to oganesson's high electropositivity.[89] Oganesson is predicted to be sufficiently electropositive[89] to form an Og–Cl bond with chlorine.[70]
A compound of oganesson and tennessine, OgTs4, has been predicted to be potentially stable chemically.[90]
See also
Notes
- ↑ The names einsteinium and fermium for elements 99 and 100 were proposed when their namesakes (Albert Einstein and Enrico Fermi respectively) were still alive, but were not made official until Einstein and Fermi had died.[4]
- ↑ In Russian, Oganessian's name is spelled Оганесян [ˈɐgənʲɪˈsʲan]; the transliteration in accordance with the rules of the English language would be Oganesyan, with one s. Similarly, the Russian name for the element is оганесон, letter-for-letter oganeson. Oganessian is the Russified version of the Armenian last name Hovhannisyan (Armenian: Հովհաննիսյան [hɔvhɑnnisˈjɑn]). It means "son of Hovhannes", i.e., "son of John". It is the most common surname in Armenia.
References
- ↑ "IUPAC Announces the Names of the Elements 113, 115, 117, and 118". IUPAC. 30 November 2016. https://iupac.org/iupac-announces-the-names-of-the-elements-113-115-117-and-118/.
- ↑ St. Fleur, Nicholas (1 December 2016). "Four New Names Officially Added to the Periodic Table of Elements". The New York Times. https://www.nytimes.com/2016/12/01/science/periodic-table-new-elements.html.
- ↑ 3.0 3.1 3.2 "IUPAC Is Naming The Four New Elements Nihonium, Moscovium, Tennessine, And Oganesson". IUPAC. 8 June 2016. https://iupac.org/iupac-is-naming-the-four-new-elements-nihonium-moscovium-tennessine-and-oganesson/.
- ↑ Hoffman, Ghiorso & Seaborg 2000, pp. 187–189.
- ↑ 5.0 5.1 5.2 Smits, Odile R.; Mewes, Jan-Michael; Jerabek, Paul; Schwerdtfeger, Peter (2020). "Oganesson: A Noble Gas Element That Is Neither Noble Nor a Gas" (PDF). Angewandte Chemie International Edition 59 (52): 23636–23640. doi:10.1002/anie.202011976. PMC 7814676. https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.202011976. Retrieved 2023-10-23.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Cite error: Invalid
<ref>tag; no text was provided for refs namedNash2005 - ↑ Kragh 2018, p. 6.
- ↑ Leach, Mark R.. "The INTERNET Database of Periodic Tables". https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=285.
- ↑ 9.0 9.1 9.2 Cite error: Invalid
<ref>tag; no text was provided for refs named60s - ↑ 10.0 10.1 10.2 Fricke, Burkhard (1975). "Superheavy elements: a prediction of their chemical and physical properties". Recent Impact of Physics on Inorganic Chemistry. Structure and Bonding 21: 89–144. doi:10.1007/BFb0116498. ISBN 978-3-540-07109-9. https://www.researchgate.net/publication/225672062. Retrieved 4 October 2013.
- ↑ Pitzer, Kenneth (1975). "Are elements 112, 114, and 118 relatively inert gases?". The Journal of Chemical Physics 2 (63): 1032–1033. doi:10.1063/1.431398. https://escholarship.org/uc/item/2qw742ss.
- ↑ 12.0 12.1 Smolanczuk, R. (1999). "Production mechanism of superheavy nuclei in cold fusion reactions". Physical Review C 59 (5): 2634–2639. doi:10.1103/PhysRevC.59.2634. Bibcode: 1999PhRvC..59.2634S.
- ↑ Ninov, Viktor (1999). "Observation of Superheavy Nuclei Produced in the Reaction of 86Kr with 208Pb". Physical Review Letters 83 (6): 1104–1107. doi:10.1103/PhysRevLett.83.1104. Bibcode: 1999PhRvL..83.1104N. https://zenodo.org/record/1233919. Template:Retraction
- ↑ Service, R. F. (1999). "Berkeley Crew Bags Element 118". Science 284 (5421): 1751. doi:10.1126/science.284.5421.1751.
- ↑ Public Affairs Department, Lawrence Berkeley Laboratory (21 July 2001). "Results of element 118 experiment retracted". https://enews.lbl.gov/Science-Articles/Archive/118-retraction.html.
- ↑ Dalton, R. (2002). "Misconduct: The stars who fell to Earth". Nature 420 (6917): 728–729. doi:10.1038/420728a. PMID 12490902. Bibcode: 2002Natur.420..728D.
- ↑ "Element 118 disappears two years after it was discovered" (in en-GB). 2001-08-02. https://physicsworld.com/a/element-118-disappears-two-years-after-it-was-discovered/.
- ↑ Zagrebaev, Karpov & Greiner 2013.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 Cite error: Invalid
<ref>tag; no text was provided for refs namedsynthesis-118-116 - ↑ Oganessian, Yu. T. (2002). "Results from the first 249Cf+48Ca experiment". JINR Communication. https://www.jinr.ru/publish/Preprints/2002/287(D7-2002-287)e.pdf. Retrieved 13 June 2009.
- ↑ 21.0 21.1 Moody, Ken (30 November 2013). "Synthesis of Superheavy Elements". in Schädel, Matthias; Shaughnessy, Dawn. The Chemistry of Superheavy Elements (2nd ed.). Springer Science & Business Media. pp. 24–8. ISBN 9783642374661.
- ↑ Oganessian, Yu. T. (2002). "Element 118: results from the first 249Cf + 48Ca experiment". Communication of the Joint Institute for Nuclear Research. https://159.93.28.88/linkc/118/anno.html.
- ↑ "Livermore scientists team with Russia to discover element 118". Livermore press release. 3 December 2006. https://www.llnl.gov/news/newsreleases/2006/NR-06-10-03.html.
- ↑ Oganessian, Yu. T. (2006). "Synthesis and decay properties of superheavy elements". Pure Appl. Chem. 78 (5): 889–904. doi:10.1351/pac200678050889.
- ↑ Sanderson, K. (2006). "Heaviest element made – again". Nature News. doi:10.1038/news061016-4.
- ↑ Schewe, P.; Stein, B. (17 October 2006). "Elements 116 and 118 Are Discovered". Physics News Update. American Institute of Physics. https://www.aip.org/pnu/2006/797.html.
- ↑ Weiss, R. (17 October 2006). "Scientists Announce Creation of Atomic Element, the Heaviest Yet". The Washington Post. https://www.washingtonpost.com/wp-dyn/content/article/2006/10/16/AR2006101601083.html.
- ↑ Barber, Robert C.; Karol, Paul J.; Nakahara, Hiromichi; Vardaci, Emanuele; Vogt, Erich W. (2011). "Discovery of the elements with atomic numbers greater than or equal to 113 (IUPAC Technical Report)". Pure and Applied Chemistry 83 (7): 1. doi:10.1351/PAC-REP-10-05-01.
- ↑ "Oganesson". WebElements Periodic Table. https://www.webelements.com/oganesson/.
- ↑ Jacoby, Mitch (17 October 2006). "Element 118 Detected, With Confidence". Chemical & Engineering News 84 (43): 11. doi:10.1021/cen-v084n043.p011. https://pubs.acs.org/cen/news/84/i43/8443element118.html. Retrieved 18 January 2008. ""I would say we're very confident."".
- ↑ Discovery and Assignment of Elements with Atomic Numbers 113, 115, 117 and 118. IUPAC (30 December 2015)
- ↑ Karol, Paul J.; Barber, Robert C.; Sherrill, Bradley M.; Vardaci, Emanuele; Yamazaki, Toshimitsu (29 December 2015). "Discovery of the element with atomic number Z = 118 completing the 7th row of the periodic table (IUPAC Technical Report)". Pure Appl. Chem. 88 (1–2): 155–160. doi:10.1515/pac-2015-0501. https://zenodo.org/record/6472870.
- ↑ 33.0 33.1 Voinov, A. A.; Oganessian, Yu. Ts; Abdullin, F. Sh.; Brewer, N. T.; Dmitriev, S. N.; Grzywacz, R. K.; Hamilton, J. H.; Itkis, M. G. et al. (2016). "Results from the Recent Study of the 249–251Cf + 48Ca Reactions". in Peninozhkevich, Yu. E.; Sobolev, Yu. G.. Exotic Nuclei. pp. 219–223. ISBN 9789813226555.
- ↑ Sychev, Vladimir (8 February 2017). "Юрий Оганесян: мы хотим узнать, где кончается таблица Менделеева" (in ru). RIA Novosti. https://ria.ru/interview/20170208/1487412085.html.
- ↑ Roberto, J. B. (31 March 2015). "Actinide Targets for Super-Heavy Element Research". Texas A & M University. https://cyclotron.tamu.edu/she2015/assets/pdfs/presentations/Roberto_SHE_2015_TAMU.pdf.
- ↑ Hauschild, K. (26 June 2019). "Superheavy nuclei at RIKEN, Dubna, and JYFL". Conseil Scientifique de l'IN2P3. https://in2p3.cnrs.fr/sites/institut_in2p3/files/page/2019-07/6-Pres-HAUSCHILD_-compresse%CC%81.pdf. Retrieved 31 July 2019.
- ↑ Hauschild, K. (2019). "Heavy nuclei at RIKEN, Dubna, and JYFL". Conseil Scientifique de l'IN2P3. https://in2p3.cnrs.fr/sites/institut_in2p3/files/page/2019-07/6-Doc-HAUSCHILD-comp.pdf. Retrieved 1 August 2019.
- ↑ Chatt, J. (1979). "Recommendations for the Naming of Elements of Atomic Numbers Greater than 100". Pure Appl. Chem. 51 (2): 381–384. doi:10.1351/pac197951020381.
- ↑ Wieser, M.E. (2006). "Atomic weights of the elements 2005 (IUPAC Technical Report)". Pure Appl. Chem. 78 (11): 2051–2066. doi:10.1351/pac200678112051.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedHaire - ↑ "Discovery of New Elements Makes Front Page News". Berkeley Lab Research Review Summer 1999. 1999. https://lbl.gov/Science-Articles/Research-Review/Magazine/1999/departments/breaking_news.shtml.
- ↑ Koppenol, W. H. (2002). "Naming of new elements (IUPAC Recommendations 2002)". Pure and Applied Chemistry 74 (5): 787. doi:10.1351/pac200274050787. https://media.iupac.org/publications/pac/2002/pdf/7405x0787.pdf.
- ↑ "New chemical elements discovered in Russia's Science City". 12 February 2007. https://news.rin.ru/eng/news/9886/9/6/.
- ↑ Yemel'yanova, Asya (17 December 2006). "118-й элемент назовут по-русски (118th element will be named in Russian)" (in ru). vesti.ru. https://www.vesti.ru/doc.html?id=113947.
- ↑ "Российские физики предложат назвать 116 химический элемент московием (Russian Physicians Will Suggest to Name Element 116 Moscovium)" (in ru). rian.ru. 2011. https://ria.ru/science/20110326/358081075.html.
- ↑ "News: Start of the Name Approval Process for the Elements of Atomic Number 114 and 116". International Union of Pure and Applied Chemistry. https://www.iupac.org/news/news-detail/article/start-of-the-name-approval-process-for-the-elements-of-atomic-number-114-and-116.html.
- ↑ Koppenol, W. H. (2002). "Naming of new elements (IUPAC Recommendations 2002)". Pure and Applied Chemistry 74 (5): 787–791. doi:10.1351/pac200274050787. https://media.iupac.org/publications/pac/2002/pdf/7405x0787.pdf.
- ↑ Koppenol, Willem H.; Corish, John; García-Martínez, Javier; Meija, Juris; Reedijk, Jan (2016). "How to name new chemical elements (IUPAC Recommendations 2016)". Pure and Applied Chemistry 88 (4): 401–405. doi:10.1515/pac-2015-0802. https://doc.rero.ch/record/325660/files/pac-2015-0802.pdf.
- ↑ "What it takes to make a new element". Chemistry World. https://www.chemistryworld.com/what-it-takes-to-make-a-new-element/1017677.article.
- ↑ 50.0 50.1 Tarasevich, Grigoriy; Lapenko, Igor (2019). "Юрий Оганесян о тайнах ядра, новых элементах и смысле жизни" (in ru). Kot Shryodingyera (Direktsiya Festivalya Nauki) (Special): 22.
- ↑ 51.0 51.1 Gray, Richard (11 April 2017). "Mr Element 118: The only living person on the periodic table". New Scientist. https://www.newscientist.com/article/mg23431210-600-up-and-atom-breaking-the-periodic-table/.
- ↑ Fedorova, Vera (3 March 2017). "At the inauguration ceremony of the new elements of the Periodic table of D.I. Mendeleev". Joint Institute for Nuclear Research. https://www.jinr.ru/posts/at-the-inauguration-ceremony-of-the-new-elements-of-the-periodic-table-of-d-i-mendeleev/.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBloomberg - ↑ de Marcillac, P.; Coron, N.; Dambier, G. et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature 422 (6934): 876–878. doi:10.1038/nature01541. PMID 12712201. Bibcode: 2003Natur.422..876D.
- ↑ Möller, P. (2016). "The limits of the nuclear chart set by fission and alpha decay". EPJ Web of Conferences 131: 03002:1–8. doi:10.1051/epjconf/201613103002. Bibcode: 2016EPJWC.13103002M. https://inspirehep.net/record/1502715/files/epjconf-NS160-03002.pdf.
- ↑ Considine, G. D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9th ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096.
- ↑ Oganessian, Yu. Ts.; Sobiczewski, A.; Ter-Akopian, G. M. (9 January 2017). "Superheavy nuclei: from predictions to discovery". Physica Scripta 92 (2): 023003–1–21. doi:10.1088/1402-4896/aa53c1. Bibcode: 2017PhyS...92b3003O.
- ↑ "Oganesson - Element information, properties and uses | Periodic Table". https://www.rsc.org/periodic-table/element/118/Oganesson.
- ↑ "Oganesson - Protons - Neutrons - Electrons - Electron Configuration" (in en-US). 2020-12-08. https://material-properties.org/oganesson-protons-neutrons-electrons-electron-configuration/.
- ↑ 60.0 60.1 60.2 60.3 Chowdhury, Roy P.; Samanta, C.; Basu, D. N. (2006). "α decay half-lives of new superheavy elements". Phys. Rev. C 73 (1): 014612. doi:10.1103/PhysRevC.73.014612. Bibcode: 2006PhRvC..73a4612C.
- ↑ Oganessian, Yu. T. (2007). "Heaviest nuclei from 48Ca-induced reactions". Journal of Physics G: Nuclear and Particle Physics 34 (4): R165–R242. doi:10.1088/0954-3899/34/4/R01. Bibcode: 2007JPhG...34R.165O.
- ↑ Chowdhury, Roy P.; Samanta, C.; Basu, D. N. (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Physical Review C 77 (4): 044603. doi:10.1103/PhysRevC.77.044603. Bibcode: 2008PhRvC..77d4603C.
- ↑ Chowdhury, R. P.; Samanta, C.; Basu, D.N. (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". Atomic Data and Nuclear Data Tables 94 (6): 781–806. doi:10.1016/j.adt.2008.01.003. Bibcode: 2008ADNDT..94..781C.
- ↑ 64.0 64.1 Royer, G.; Zbiri, K.; Bonilla, C. (2004). "Entrance channels and alpha decay half-lives of the heaviest elements". Nuclear Physics A 730 (3–4): 355–376. doi:10.1016/j.nuclphysa.2003.11.010. Bibcode: 2004NuPhA.730..355R.
- ↑ Duarte, S. B.; Tavares, O. A. P.; Gonçalves, M.; Rodríguez, O.; Guzmán, F.; Barbosa, T. N.; García, F.; Dimarco, A. (2004). "Half-life predictions for decay modes of superheavy nuclei". Journal of Physics G: Nuclear and Particle Physics 30 (10): 1487–1494. doi:10.1088/0954-3899/30/10/014. Bibcode: 2004JPhG...30.1487D. https://www.iaea.org/inis/collection/NCLCollectionStore/_Public/36/073/36073846.pdf.
- ↑ Oganessian, Yu. Ts.; Utyonkov, V.; Lobanov, Yu.; Abdullin, F.; Polyakov, A.; Shirokovsky, I.; Tsyganov, Yu.; Gulbekian, G. et al. (2004). "Measurements of cross sections and decay properties of the isotopes of elements 112, 114, and 116 produced in the fusion reactions 233,238U, 242Pu, and 248Cm+48Ca". Physical Review C 70 (6): 064609. doi:10.1103/PhysRevC.70.064609. Bibcode: 2004PhRvC..70f4609O. https://www1.jinr.ru/Preprints/2004/160(E7-2004-160).pdf.
- ↑ Samanta, C.; Chowdhury, R. P.; Basu, D.N. (2007). "Predictions of alpha decay half-lives of heavy and superheavy elements". Nucl. Phys. A 789 (1–4): 142–154. doi:10.1016/j.nuclphysa.2007.04.001. Bibcode: 2007NuPhA.789..142S.
- ↑ Bader, Richard F.W. "An Introduction to the Electronic Structure of Atoms and Molecules". McMaster University. https://miranda.chemistry.mcmaster.ca/esam/.
- ↑ "Ununoctium (Uuo) – Chemical properties, Health and Environmental effects". Lenntech. https://lenntech.com/Periodic-chart-elements/Uuo-en.htm.
- ↑ 70.0 70.1 70.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedKaldor - ↑ 71.0 71.1 Goidenko, Igor; Labzowsky, Leonti; Eliav, Ephraim; Kaldor, Uzi; Pyykkö, Pekka (2003). "QED corrections to the binding energy of the eka-radon (Z=118) negative ion". Physical Review A 67 (2): 020102(R). doi:10.1103/PhysRevA.67.020102. Bibcode: 2003PhRvA..67b0102G.
- ↑ Eliav, Ephraim; Kaldor, Uzi; Ishikawa, Y.; Pyykkö, P. (1996). "Element 118: The First Rare Gas with an Electron Affinity". Physical Review Letters 77 (27): 5350–5352. doi:10.1103/PhysRevLett.77.5350. PMID 10062781. Bibcode: 1996PhRvL..77.5350E.
- ↑ Landau, Arie; Eliav, Ephraim; Ishikawa, Yasuyuki; Kador, Uzi (25 May 2001). "Benchmark calculations of electron affinities of the alkali atoms sodium to eka-francium (element 119)". Journal of Chemical Physics 115 (6): 2389–92. doi:10.1063/1.1386413. Bibcode: 2001JChPh.115.2389L. https://www.researchgate.net/publication/234859102. Retrieved 15 September 2015.
- ↑ Borschevsky, Anastasia; Pershina, Valeria; Kaldor, Uzi; Eliav, Ephraim. "Fully relativistic ab initio studies of superheavy elements". Johannes Gutenberg University Mainz. https://www.kernchemie.uni-mainz.de/downloads/che_7/presentations/borschevsky.pdf.
- ↑ Borschevsky, Anastasia; Pershina, Valeria; Eliav, Ephraim; Kaldor, Uzi (27 August 2009). "Electron affinity of element 114, with comparison to Sn and Pb". Chemical Physics Letters 480 (1): 49–51. doi:10.1016/j.cplett.2009.08.059. Bibcode: 2009CPL...480...49B.
- ↑ 76.0 76.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedIPEA - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedoganesson-melting - ↑ Nash, Clinton S.; Bursten, Bruce E. (1999). "Spin-Orbit Effects, VSEPR Theory, and the Electronic Structures of Heavy and Superheavy Group IVA Hydrides and Group VIIIA Tetrafluorides. A Partial Role Reversal for Elements 114 and 118". Journal of Physical Chemistry A 1999 (3): 402–410. doi:10.1021/jp982735k. PMID 27676357. Bibcode: 1999JPCA..103..402N.
- ↑ 79.0 79.1 Jerabek, Paul; Schuetrumpf, Bastian; Schwerdtfeger, Peter; Nazarewicz, Witold (2018). "Electron and Nucleon Localization Functions of Oganesson: Approaching the Thomas-Fermi Limit". Phys. Rev. Lett. 120 (5): 053001. doi:10.1103/PhysRevLett.120.053001. PMID 29481184. Bibcode: 2018PhRvL.120e3001J.
- ↑ Schuetrumpf, B.; Nazarewicz, W.; Reinhard, P.-G. (2017-08-11). "Central depression in nucleonic densities: Trend analysis in the nuclear density functional theory approach". Physical Review C 96 (2): 024306. doi:10.1103/PhysRevC.96.024306. Bibcode: 2017PhRvC..96b4306S. https://link.aps.org/doi/10.1103/PhysRevC.96.024306.
- ↑ Garisto, Dan (12 February 2018). "5 ways the heaviest element on the periodic table is really bizarre" (in en-US). https://www.sciencenews.org/article/5-ways-heaviest-element-periodic-table-really-bizarre.
- ↑ Mewes, Jan-Michael; Smits, Odile Rosette; Jerabek, Paul; Schwerdtfeger, Peter (25 July 2019). "Oganesson is a Semiconductor: On the Relativistic Band‐Gap Narrowing in the Heaviest Noble‐Gas Solids". Angewandte Chemie 58 (40): 14260–14264. doi:10.1002/anie.201908327. PMID 31343819.
- ↑ "Oganesson: Compounds Information". WebElements Periodic Table. https://www.webelements.com/oganesson/compounds.html.
- ↑ 84.0 84.1 84.2 84.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedhydride - ↑ 85.0 85.1 Han, Young-Kyu; Lee, Yoon Sup (1999). "Structures of RgFn (Rg = Xe, Rn, and Element 118. n = 2, 4.) Calculated by Two-component Spin-Orbit Methods. A Spin-Orbit Induced Isomer of (118)F4". Journal of Physical Chemistry A 103 (8): 1104–1108. doi:10.1021/jp983665k. Bibcode: 1999JPCA..103.1104H.
- ↑ Liebman, Joel F. (1975). "Conceptual Problems in Noble Gas and Fluorine Chemistry, II: The Nonexistence of Radon Tetrafluoride". Inorg. Nucl. Chem. Lett. 11 (10): 683–685. doi:10.1016/0020-1650(75)80185-1.
- ↑ Seppelt, Konrad (2015). "Molecular Hexafluorides". Chemical Reviews 115 (2): 1296–1306. doi:10.1021/cr5001783. PMID 25418862.
- ↑ Pitzer, Kenneth S. (1975). "Fluorides of radon and element 118". Journal of the Chemical Society, Chemical Communications (18): 760–761. doi:10.1039/C3975000760b. https://escholarship.org/content/qt8xz4g1ff/qt8xz4g1ff.pdf?t=p2at3t.
- ↑ 89.0 89.1 Seaborg, Glenn Theodore (c. 2006). "transuranium element (chemical element)". Britannica Online. https://www.britannica.com/EBchecked/topic/603220/transuranium-element. Retrieved 16 March 2010.
- ↑ Loveland, Walter (1 June 2021). "Relativistic effects for the superheavy reaction Og + 2Ts2 → OgTs4 (Td or D4h): dramatic relativistic effects for atomization energy of superheavy Oganesson tetratennesside OgTs4 and prediction of the existence of tetrahedral OgTs4". Theoretical Chemistry Accounts 140 (75). doi:10.1007/s00214-021-02777-2. https://link.springer.com/article/10.1007/s00214-021-02777-2. Retrieved 30 June 2021.
Bibliography
- Audi, G.; Kondev, F. G.; Wang, M. et al. (2017). "The NUBASE2016 evaluation of nuclear properties". Chinese Physics C 41 (3): 030001. doi:10.1088/1674-1137/41/3/030001. Bibcode: 2017ChPhC..41c0001A.
- Beiser, A. (2003). Concepts of modern physics (6th ed.). McGraw-Hill. ISBN 978-0-07-244848-1. OCLC 48965418.
- Hoffman, D. C.; Ghiorso, A.; Seaborg, G. T. (2000). The Transuranium People: The Inside Story. World Scientific. ISBN 978-1-78-326244-1.
- Kragh, H. (2018). From Transuranic to Superheavy Elements: A Story of Dispute and Creation. Springer. ISBN 978-3-319-75813-8.
- Zagrebaev, V.; Karpov, A.; Greiner, W. (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?". Journal of Physics 420 (1): 012001. doi:10.1088/1742-6596/420/1/012001. ISSN 1742-6588. Bibcode: 2013JPhCS.420a2001Z.
Further reading
- Scerri, Eric (2007). The Periodic Table, Its Story and Its Significance. New York: Oxford University Press. ISBN 978-0-19-530573-9. https://archive.org/details/periodictableits0000scer.
External links
- 5 ways the heaviest element on the periodic table is really bizarre, ScienceNews.org
- Element 118: Experiments on discovery, archive of discoverers' official web page
- Element 118, Heaviest Ever, Reported for 1,000th of a Second, The New York Times .
- It's Elemental: Oganesson
- Oganesson at The Periodic Table of Videos (University of Nottingham)
- On the Claims for Discovery of Elements 110, 111, 112, 114, 116, and 118 (IUPAC Technical Report)
- WebElements: Oganesson
Lua error in package.lua at line 80: module 'Module:Portal/images/r' not found.
 |