Chemistry:Allopumiliotoxin 267A
From HandWiki
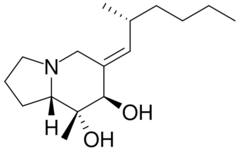
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6E,7R,8R,8aS)-8-Methyl-6-[(2R)-2-methylhexylidene]octahydroindolizine-7,8-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H29NO2 | |
| Molar mass | 267.413 g·mol−1 |
| Hazards | |
| Main hazards | Highly toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Allopumiliotoxin 267A is a toxin found in the skin of several poison frogs of the family Dendrobates.[1] It is a member of the class of compounds known as allopumiliotoxins. The frogs produce the toxin by modifying the original version, pumiliotoxin 251D.[2] It has been tested on mice and found to be five times more potent than the former version. It has been produced synthetically through a variety of different routes.[3][4][5][6]
See also
References
- ↑ Edwards, M. W.; Daly, J. W.; Myers, C. W. (1988). "Alkaloids from a Panamanian poison frog, Dendrobates speciosus: Identification of pumiliotoxin-A and allopumiliotoxin class alkaloids, 3,5-disubstituted indolizidines, 5-substituted 8-methylindolizidines, and a 2-methyl-6-nonyl-4-hydroxypiperidine". Journal of Natural Products 51 (6): 1188–97. doi:10.1021/np50060a023. PMID 3236011.
- ↑ Daly, J. W.; Garraffo, H. M.; Spande, T. F.; Clark, V. C.; Ma, J.; Ziffer, H.; Cover Jr, J. F. (2003). "Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid poison frogs of the genus Dendrobates". Proceedings of the National Academy of Sciences of the United States of America 100 (19): 11092–7. doi:10.1073/pnas.1834430100. PMID 12960405. Bibcode: 2003PNAS..10011092D.
- ↑ Comins, D. L.; Huang, S.; McArdle, C. L.; Ingalls, C. L. (2001). "Enantiopure 2,3-dihydro-4-pyridones as synthetic intermediates: A concise asymmetric synthesis of (+)-allopumiliotoxin 267A". Organic Letters 3 (3): 469–71. doi:10.1021/ol0069709. PMID 11428041.
- ↑ Franklin, Alison S.; Overman, Larry E. (1996). "Total Syntheses of Pumiliotoxin a and Allopumiliotoxin Alkaloids. Interplay of Pharmacologically Active Natural Products and New Synthetic Methods and Strategies". Chemical Reviews 96 (1): 505–522. doi:10.1021/cr950021p. PMID 11848762.
- ↑ Tang, Xiao-Qing; Montgomery, John (2000). "Nickel-Catalyzed Preparation of Bicyclic Heterocycles: Total Synthesis of (+)-Allopumiliotoxin 267A, (+)-Allopumiliotoxin 339A, and (+)-Allopumiliotoxin 339B". Journal of the American Chemical Society 122 (29): 6950–6954. doi:10.1021/ja001440t. https://figshare.com/articles/Nickel-Catalyzed_Preparation_of_Bicyclic_Heterocycles_Total_Synthesis_of_-Allopumiliotoxin_267A_-Allopumiliotoxin_339A_and_-Allopumiliotoxin_339B/3629484.
- ↑ Aoyagi, Sakae; Wang, Tzu Chueh; Kibayashi, Chihiro (1993). "Highly stereoselective total syntheses of (+)-allopumiliotoxins 267A and 339A via intramolecular nickel(II)/Chromium(II)-mediated cyclization". Journal of the American Chemical Society 115 (24): 11393–11409. doi:10.1021/ja00077a044.
 |

