Chemistry:Hyponitrous acid
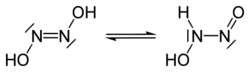
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Diazenediol | |||
| Systematic IUPAC name
N-(Hydroxyimino)hydroxylamine | |||
| Other names
Hyponitrous acid dimer
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChemSpider | |||
| 141300 | |||
| KEGG | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| H2N2O2 | |||
| Molar mass | 62.0282 g/mol | ||
| Appearance | white crystals | ||
| Conjugate base | Hyponitrite | ||
| Hazards | |||
| Main hazards | explosive when dry | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
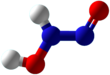
Hyponitrous acid is a chemical compound with formula H2N2O2 or HON=NOH. It is an isomer of nitramide, H2N−NO2; and a formal dimer of azanone, HNO.
Hyponitrous acid forms two series of salts, the hyponitrites containing the [ON=NO]2− anion, and the "acid hyponitrites" containing the [HON=NO]− anion.[1]
Structure and properties
There are two possible structures of hyponitrous acid, trans and cis. trans-Hyponitrous acid forms white crystals that are explosive when dry. In aqueous solution, it is a weak acid (pKa1 = 7.21, pKa2 = 11.54), and decomposes to nitrous oxide and water with a half life of 16 days at 25 °C at pH 1–3:
- [math]\ce{ H2N2O2 -> H2O + N2O }[/math]
Since this reaction is not reversible, N2O should not be considered as the anhydride of H2N2O2.[1]
The cis acid is not known,[1] but its sodium salt can be obtained.[2]
Preparation
Hyponitrous acid (trans) can be prepared from silver(I) hyponitrite and anhydrous HCl in ether:
- [math]\ce{ Ag2N2O2 + 2 HCl -> H2N2O2 + 2 AgCl }[/math]
Spectroscopic data indicate a trans configuration for the resulting acid.[2]
It can also be synthesized from hydroxylamine and nitrous acid:
- [math]\ce{ NH2OH + HNO2 -> H2N2O2 + H2O }[/math]
Biological aspects
In enzymology, a hyponitrite reductase is an enzyme that catalyzes the chemical reaction[3]
- [math]\ce{ H2N2O2 + 2 NADH + 2 H+ <-> 2 NH2OH + 2 NAD+ }[/math]
References
- ↑ 1.0 1.1 1.2 Wiberg, Egon; Holleman, Arnold Frederick (2001). Inorganic Chemistry. Elsevier. ISBN 0-12-352651-5.
- ↑ 2.0 2.1 Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 468. ISBN 978-0-13-175553-6. https://archive.org/details/inorganicchemist00hous_159.
- ↑ "ENZYME - 1.7.1.5 Hyponitrite reductase". http://enzyme.expasy.org/EC/1.7.1.5.
 |