Biology:Phospholipase D1
 Generic protein structure example |
Phospholipase D1 (PLD1) is an enzyme that in humans is encoded by the PLD1 gene,[1][2] though analogues are found in plants, fungi, prokaryotes, and even viruses.[3]
History
The possibility of PLD1 was first mentioned in 1947 by authors Hanahan and Chaikoff at Berkeley when describing a carrot enzyme that could "[split] choline from phospholipids."[4] PLD was first derived in mammals in 1975 by Saito and Kanfer, who noted its activity in rats.[5] PLD was first cloned from HeLa cell cDNA in 1995, while mammalian PLD1 was first cloned from a rat in 1997.[3]
Function
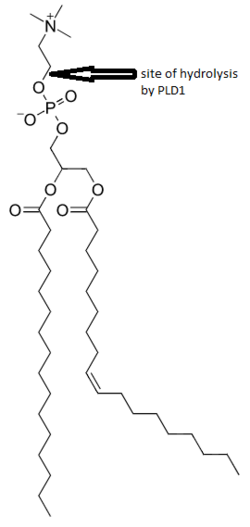
Phosphatidylcholine (PC)-specific phospholipases D (PLDs) EC 3.1.4.4 catalyze the hydrolysis of PC to produce phosphatidic acid (PA) and choline. A range of agonists acting through G protein-coupled receptors and receptor tyrosine kinases stimulate this hydrolysis. PC-specific PLD activity has been implicated in numerous cellular pathways, including membrane trafficking, signal transduction, platelet coagulation, mitosis, apoptosis, and the creation of cytoplasmic lipid droplets.[2][3][6][7]
Membrane trafficking
PLD1 has been shown to associate at the plasma membrane, late endosome,[8] early endosome, and the Golgi apparatus.[3][5] There is evidence that PA is able to assist in negative membrane curvature due to its head group being smaller than in many other lipids.[3] One experiment with PLD1 knockout showed a significant reduction in the number of exocytotic fusion events, implying a strong role in exocytosis.[9]
Signal transduction
PLD1 may play a role in some cells in the endocytosis of signaling receptors or exocytosis of signaling molecules. For example, one experiment in B cells showed that limiting PLD1 led to significantly reduced endocytosis of the B cell receptor.[8] Another experiment showed that knocking out PLD1 may hinder the ability of mice to secrete catecholamines, molecules that are essential for vesicular communication across the body.[9]
Structure
Mammalian PLD1 has several domains for activators, inhibitors, and catalysis, which it shares with PLD2. Domains for both activation and inhibition are referred to as the phox homology (PX) and pleckstrin homology (PH) domains. The catalytic domain consists of two HKD regions, so named for three of the amino acids that are key in catalysis. These domains are conserved across many organisms.[3][5] There are two splice variants of the protein, PLD1a and PLD1b, but they do not seem to localize any differently.[3]
Applications
Alzheimer's disease: PA, which is produced in part by PLD1, seems to be involved in the movement of β-amyloid, which could precede amyloidogenesis.[10]
Cancer: certain rat tumors with dominant negative PLD do not appear to form new colonies or tumors.[3][10]
Thrombosis: PLD knockout mice appear to have reduced occlusion, thus offsetting thrombosis.[3]
Type II Diabetes: the protein PED/PEA15 is often elevated in type II diabetic patients, thus enhancing PLD1 activity, and in turn impairing insulin.[3]
Interactions
Phospholipase D1 has been shown to interact with:
- Alpha-synuclein,[11]
- Amphiphysin,[12]
- BIN1,[12]
- CDC42,[13]
- PEA15,[14]
- Protein kinase N1[15]
- RALA,[16][17] and
- RHOA.[18][19]
Inhibitors
- Calphostin-c, an inhibitor[3]
- VU-0359595: 1,700-fold selective versus phospholipase D2, IC50 = 3.7nM.[20]
References
- ↑ "Assignment of human PLD1 to human chromosome band 3q26 by fluorescence in situ hybridization". Cytogenetics and Cell Genetics 82 (3–4): 224. February 1999. doi:10.1159/000015105. PMID 9858822.
- ↑ 2.0 2.1 "Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family". The Journal of Biological Chemistry 270 (50): 29640–3. December 1995. doi:10.1074/jbc.270.50.29640. PMID 8530346.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 "Phospholipase D: enzymology, functionality, and chemical modulation". Chemical Reviews 111 (10): 6064–119. October 2011. doi:10.1021/cr200296t. PMID 21936578.
- ↑ "The phosphorus-containing lipides of the carrot". The Journal of Biological Chemistry 168 (1): 233–40. April 1947. doi:10.1016/S0021-9258(17)35110-4. PMID 20291081. http://www.jbc.org/content/168/1/233.
- ↑ 5.0 5.1 5.2 "Phospholipase D: a lipid centric review". Cellular and Molecular Life Sciences 62 (19–20): 2305–16. October 2005. doi:10.1007/s00018-005-5195-z. PMID 16143829.
- ↑ "Entrez Gene: PLD1 phospholipase D1, phosphatidylcholine-specific". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5337.
- ↑ "PLD1 and ERK2 regulate cytosolic lipid droplet formation". Journal of Cell Science 119 (Pt 11): 2246–57. June 2006. doi:10.1242/jcs.02941. PMID 16723731.
- ↑ 8.0 8.1 "Phospholipase D in endocytosis and endosomal recycling pathways". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1791 (9): 845–9. September 2009. doi:10.1016/j.bbalip.2009.05.011. PMID 19540357.
- ↑ 9.0 9.1 "Mono- and Poly-unsaturated Phosphatidic Acid Regulate Distinct Steps of Regulated Exocytosis in Neuroendocrine Cells". Cell Reports 32 (7): 108026. August 2020. doi:10.1016/j.celrep.2020.108026. PMID 32814056.
- ↑ 10.0 10.1 "Regulation of Membrane Turnover by Phosphatidic Acid: Cellular Functions and Disease Implications". Frontiers in Cell and Developmental Biology 7: 83. 2019-06-04. doi:10.3389/fcell.2019.00083. PMID 31231646.
- ↑ "alpha-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells". The Journal of Biological Chemistry 277 (14): 12334–42. April 2002. doi:10.1074/jbc.M110414200. PMID 11821392.
- ↑ 12.0 12.1 "Inhibition of phospholipase D by amphiphysins". The Journal of Biological Chemistry 275 (25): 18751–8. June 2000. doi:10.1074/jbc.M001695200. PMID 10764771.
- ↑ "Activation of phospholipase D1 by Cdc42 requires the Rho insert region". The Journal of Biological Chemistry 275 (21): 15665–8. May 2000. doi:10.1074/jbc.M000076200. PMID 10747870.
- ↑ "Regulation of expression of phospholipase D1 and D2 by PEA-15, a novel protein that interacts with them". The Journal of Biological Chemistry 275 (45): 35224–32. November 2000. doi:10.1074/jbc.M003329200. PMID 10926929.
- ↑ "PKN regulates phospholipase D1 through direct interaction". The Journal of Biological Chemistry 276 (21): 18096–101. May 2001. doi:10.1074/jbc.M010646200. PMID 11259428.
- ↑ "RalA interacts directly with the Arf-responsive, PIP2-dependent phospholipase D1". Biochemical and Biophysical Research Communications 235 (3): 854–9. June 1997. doi:10.1006/bbrc.1997.6793. PMID 9207251.
- ↑ "Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA". FEBS Letters 430 (3): 231–5. July 1998. doi:10.1016/s0014-5793(98)00661-9. PMID 9688545.
- ↑ "Activation of phospholipase D1 by ADP-ribosylated RhoA". Biochemical and Biophysical Research Communications 302 (1): 127–32. February 2003. doi:10.1016/s0006-291x(03)00112-8. PMID 12593858.
- ↑ "Determination of interaction sites of phospholipase D1 for RhoA". The Biochemical Journal 355 (Pt 3): 779–85. May 2001. doi:10.1042/bj3550779. PMID 11311142.
- ↑ "Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privileged structures for PLD1 specificity". Bioorganic & Medicinal Chemistry Letters 19 (7): 1916–20. April 2009. doi:10.1016/j.bmcl.2009.02.057. PMID 19268584.
Further reading
- "Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-alpha". The Journal of Biological Chemistry 272 (6): 3860–8. February 1997. doi:10.1074/jbc.272.6.3860. PMID 9013646.
- "RalA interacts directly with the Arf-responsive, PIP2-dependent phospholipase D1". Biochemical and Biophysical Research Communications 235 (3): 854–9. June 1997. doi:10.1006/bbrc.1997.6793. PMID 9207251.
- "Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization". Current Biology 7 (3): 191–201. March 1997. doi:10.1016/S0960-9822(97)70090-3. PMID 9395408.
- "Determination of interaction sites on the small G protein RhoA for phospholipase D". The Journal of Biological Chemistry 273 (19): 11596–604. May 1998. doi:10.1074/jbc.273.19.11596. PMID 9565577.
- "Cloning and initial characterization of a human phospholipase D2 (hPLD2). ADP-ribosylation factor regulates hPLD2". The Journal of Biological Chemistry 273 (21): 12846–52. May 1998. doi:10.1074/jbc.273.21.12846. PMID 9582313.
- "Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA". FEBS Letters 430 (3): 231–5. July 1998. doi:10.1016/S0014-5793(98)00661-9. PMID 9688545.
- "Characterization of human PLD2 and the analysis of PLD isoform splice variants". FASEB Journal 12 (13): 1309–17. October 1998. doi:10.1096/fasebj.12.13.1309. PMID 9761774.
- "Phospholipase D1 in caveolae: regulation by protein kinase Calpha and caveolin-1". Biochemistry 38 (12): 3763–9. March 1999. doi:10.1021/bi982478+. PMID 10090765.
- "Phosphorylation and activation of phospholipase D1 by protein kinase C in vivo: determination of multiple phosphorylation sites". Biochemistry 38 (32): 10344–51. August 1999. doi:10.1021/bi990579h. PMID 10441128.
- "Activation of phospholipase D1 by Cdc42 requires the Rho insert region". The Journal of Biological Chemistry 275 (21): 15665–8. May 2000. doi:10.1074/jbc.M000076200. PMID 10747870.
- "Involvement of Ras and Ral in chemotactic migration of skeletal myoblasts". Molecular and Cellular Biology 20 (13): 4658–65. July 2000. doi:10.1128/MCB.20.13.4658-4665.2000. PMID 10848592.
- "Regulation of expression of phospholipase D1 and D2 by PEA-15, a novel protein that interacts with them". The Journal of Biological Chemistry 275 (45): 35224–32. November 2000. doi:10.1074/jbc.M003329200. PMID 10926929.
- "Interaction of the type Ialpha PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4, 5-bisphosphate in the regulation of PLD2 activity". The EMBO Journal 19 (20): 5440–9. October 2000. doi:10.1093/emboj/19.20.5440. PMID 11032811.
- "PKN regulates phospholipase D1 through direct interaction". The Journal of Biological Chemistry 276 (21): 18096–101. May 2001. doi:10.1074/jbc.M010646200. PMID 11259428.
- "Determination of interaction sites of phospholipase D1 for RhoA". The Biochemical Journal 355 (Pt 3): 779–85. May 2001. doi:10.1042/bj3550779. PMID 11311142.
- "Actin directly interacts with phospholipase D, inhibiting its activity". The Journal of Biological Chemistry 276 (30): 28252–60. July 2001. doi:10.1074/jbc.M008521200. PMID 11373276.
- "Endosomal localization of phospholipase D 1a and 1b is defined by the C-termini of the proteins, and is independent of activity". The Biochemical Journal 356 (Pt 3): 727–36. June 2001. doi:10.1042/0264-6021:3560727. PMID 11389680.
- "Differential tyrosine phosphorylation of phospholipase D isozymes by hydrogen peroxide and the epidermal growth factor in A431 epidermoid carcinoma cells". Molecules and Cells 11 (3): 369–78. June 2001. PMID 11459228.
 |