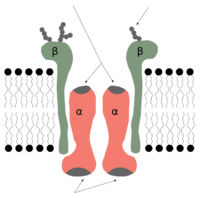
Biology:Sodium–potassium pump
| Na+ /K+ -ATPase pump | |||||||||
|---|---|---|---|---|---|---|---|---|---|
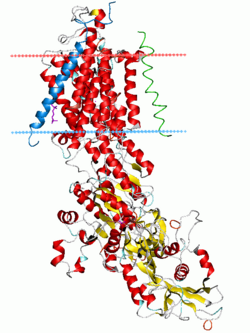 Sodium–potassium pump, E2-Pi state. Calculated hydrocarbon boundaries of the lipid bilayer are shown as blue (intracellular) and red (extracellular) planes | |||||||||
| Identifiers | |||||||||
| EC number | 7.2.2.13 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
The sodium–potassium pump (sodium–potassium adenosine triphosphatase, also known as Na+
/K+
-ATPase, Na+
/K+
pump, or sodium–potassium ATPase) is an enzyme (an electrogenic transmembrane ATPase) found in the membrane of all animal cells. It performs several functions in cell physiology.
The Na+
/K+
-ATPase enzyme is active (i.e. it uses energy from ATP). For every ATP molecule that the pump uses, three sodium ions are exported and two potassium ions are imported.[1] Thus, there is a net export of a single positive charge per pump cycle. The net effect is an extracellular concentration of sodium ions which is 5 times the intracellular concentration, and an intracellular concentration of potassium ions which is 30 times the extracellular concentration.[1]
The sodium–potassium pump was discovered in 1957 by the Danish scientist Jens Christian Skou, who was awarded a Nobel Prize for his work in 1997. Its discovery marked an important step forward in the understanding of how ions get into and out of cells, and it has particular significance for excitable cells such as nerve cells, which depend on this pump to respond to stimuli and transmit impulses.
All mammals have four different sodium pump sub-types, or isoforms. Each has unique properties and tissue expression patterns.[2] This enzyme belongs to the family of P-type ATPases.
Function
The Na+
/K+
-ATPase helps maintain resting potential, affects transport, and regulates cellular volume.[3] It also functions as a signal transducer/integrator to regulate the MAPK pathway, reactive oxygen species (ROS), as well as intracellular calcium. In fact, all cells expend a large fraction of the ATP they produce (typically 30% and up to 70% in
nerve cells) to maintain their required cytosolic Na and K
concentrations.[4]
For neurons, the Na+
/K+
-ATPase can be responsible for up to 3/4 of the cell's energy expenditure.[5] In many types of tissue, ATP consumption by the Na+
/K+
-ATPases have been related to glycolysis. This was first discovered in red blood cells (Schrier, 1966), but has later been evidenced in renal cells,[6] smooth muscles surrounding the blood vessels,[7] and cardiac purkinje cells.[8] Recently, glycolysis has also been shown to be of particular importance for Na+
/K+
-ATPase in skeletal muscles, where inhibition of glycogen breakdown (a substrate for glycolysis) leads to reduced Na+
/K+
-ATPase activity and lower force production.[9][10][11]
Resting potential
In order to maintain the cell membrane potential, cells keep a low concentration of sodium ions and high levels of potassium ions within the cell (intracellular). The sodium–potassium pump mechanism moves 3 sodium ions out and moves 2 potassium ions in, thus, in total, removing one positive charge carrier from the intracellular space (see § Mechanism for details). In addition, there is a short-circuit channel (i.e. a highly K-permeable ion channel) for potassium in the membrane, thus the voltage across the plasma membrane is close to the Nernst potential of potassium.
Reversal potential
Transport
Export of sodium ions from the cell provides the driving force for several secondary active transporters such as membrane transport proteins, which import glucose, amino acids and other nutrients into the cell by use of the sodium ion gradient.
Another important task of the Na+
-K+
pump is to provide a Na+
gradient that is used by certain carrier processes. In the gut, for example, sodium is transported out of the reabsorbing cell on the blood (interstitial fluid) side via the Na+
-K+
pump, whereas, on the reabsorbing (lumenal) side, the Na+
-glucose symporter uses the created Na+
gradient as a source of energy to import both Na+
and glucose, which is far more efficient than simple diffusion. Similar processes are located in the renal tubular system.
Controlling cell volume
Failure of the Na+
-K+
pumps can result in swelling of the cell. A cell's osmolarity is the sum of the concentrations of the various ion species and many proteins and other organic compounds inside the cell. When this is higher than the osmolarity outside of the cell, water flows into the cell through osmosis. This can cause the cell to swell up and lyse. The Na+
-K+
pump helps to maintain the right concentrations of ions.
Furthermore, when the cell begins to swell, this automatically activates the Na+
-K+
pump because it changes the internal concentrations of Na+
-K+
to which the pump is sensitive.[12]
Functioning as signal transducer
Within the last decade[when?], many independent labs have demonstrated that, in addition to the classical ion transporting, this membrane protein can also relay extracellular ouabain-binding signalling into the cell through regulation of protein tyrosine phosphorylation. For instance, a study investigated the function of Na+
/K+
-ATPase in foot muscle and hepatopancreas in land snail Otala lactea by comparing the active and estivating states.[13] They concluded that reversible phosphorylation can control the same means of coordinating ATP use by this ion pump with the rates of the ATP generation by catabolic pathways in estivating O. lactea. The downstream signals through ouabain-triggered protein phosphorylation events include activation of the mitogen-activated protein kinase (MAPK) signal cascades, mitochondrial reactive oxygen species (ROS) production, as well as activation of phospholipase C (PLC) and inositol triphosphate (IP3) receptor (IP3R) in different intracellular compartments.[14]
Protein-protein interactions play a very important role in Na+
-K+
pump-mediated signal transduction. For example, the Na+
-K+
pump interacts directly with Src, a non-receptor tyrosine kinase, to form a signaling receptor complex.[15] Src is initially inhibited by the Na+
-K+
pump. However, upon subsequent ouabain binding, the Src kinase domain is released and then activated. Based on this scenario, NaKtide, a peptide Src inhibitor derived from the Na+
-K+
pump, was developed as a functional ouabain–Na+
-K+
pump-mediated signal transduction.[16] Na+
-K+
pump also interacts with ankyrin, IP3R, PI3K, PLCgamma1 and cofilin.[17]
Controlling neuron activity states
The Na+
-K+
pump has been shown to control and set the intrinsic activity mode of cerebellar Purkinje neurons,[18] accessory olfactory bulb mitral cells[19] and probably other neuron types.[20] This suggests that the pump might not simply be a homeostatic, "housekeeping" molecule for ionic gradients, but could be a computation element in the cerebellum and the brain.[21] Indeed, a mutation in the Na+
-K+
pump causes rapid onset dystonia-parkinsonism, which has symptoms to indicate that it is a pathology of cerebellar computation.[22] Furthermore, an ouabain block of Na+
-K+
pumps in the cerebellum of a live mouse results in it displaying ataxia and dystonia.[23] Alcohol inhibits sodium–potassium pumps in the cerebellum and this is likely how it corrupts cerebellar computation and body coordination.[24][25] The distribution of the Na+
-K+
pump on myelinated axons in the human brain has been demonstrated to be along the internodal axolemma, and not within the nodal axolemma as previously thought.[26] The Na+
-K+
pump disfunction has been tied to various diseases, including epilepsy and brain malformations.[27]
Mechanism
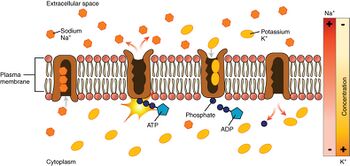
Looking at the process starting from the interior of the cell:
- The pump has a higher affinity for Na+
ions than K+
ions, thus after binding ATP, binds 3 intracellular Na+
ions.[3] - ATP is hydrolyzed, leading to phosphorylation of the pump at a highly conserved aspartate residue and subsequent release of ADP. This process leads to a conformational change in the pump.
- The conformational change exposes the Na+
ions to the extracellular region. The phosphorylated form of the pump has a low affinity for Na+
ions, so they are released; by contrast it has high affinity for the K+
ions. - The pump binds 2 extracellular K+
ions, which induces dephosphorylation of the pump, reverting it to its previous conformational state, thus releasing the K+
ions into the cell. - The unphosphorylated form of the pump has a higher affinity for Na+
ions. ATP binds, and the process starts again.
Regulation
Endogenous
The Na+
/K+
-ATPase is endogenously negatively regulated by the inositol pyrophosphate 5-InsP7, an intracellular signaling molecule generated by IP6K1, which relieves an autoinhibitory domain of PI3K p85α to drive endocytosis and degradation.[28]
The Na+
/K+
-ATPase is also regulated by reversible phosphorylation. Research has shown that in estivating animals, the Na+
/K+
-ATPase is in the phosphorylated and low activity form. Dephosphorylation of Na+
/K+
-ATPase can recover it to the high activity form.[13]
Exogenous
The Na+
/K+
-ATPase can be pharmacologically modified by administering drugs exogenously. Its expression can also be modified through hormones such as triiodothyronine, a thyroid hormone.[13][29]
For instance, Na+
/K+
-ATPase found in the membrane of heart cells is an important target of cardiac glycosides (for example digoxin and ouabain), inotropic drugs used to improve heart performance by increasing its force of contraction.
Muscle contraction is dependent on a 100- to 10,000-times-higher-than-resting intracellular Ca2+
concentration, which is caused by Ca2+
release from the muscle cells' sarcoplasmic reticulum. Immediately after muscle contraction, intracellular Ca2+
is quickly returned to its normal concentration by a carrier enzyme in the plasma membrane, and a calcium pump in sarcoplasmic reticulum, causing the muscle to relax.
According to the Blaustein-hypothesis,[30] this carrier enzyme (Na+
/Ca2+
exchanger, NCX) uses the Na gradient generated by the Na+
-K+
pump to remove Ca2+
from the intracellular space, hence slowing down the Na+
-K+
pump results in a permanently elevated Ca2+
level in the muscle, which may be the mechanism of the long-term inotropic effect of cardiac glycosides such as digoxin. The problem with this hypothesis is that at pharmacological concentrations of digitalis, less than 5% of Na/K-ATPase molecules – specifically the α2 isoform in heart and arterial smooth muscle (Kd = 32 nM) – are inhibited, not enough to affect the intracellular concentration of Na+
. However, apart from the population of Na/K-ATPase in the plasma membrane, responsible for ion transport, there is another population in the caveolae which acts as digitalis receptor and stimulates the EGF receptor.[31][32][33][34]
Pharmacological regulation
In certain conditions such as in the case of cardiac disease, the Na+
/K+
-ATPase may need to be inhibited via pharmacological means. A commonly used inhibitor used in the treatment of cardiac disease is digoxin (a cardiac glycoside) which essentially binds "to the extracellular part of enzyme i.e. that binds potassium, when it is in a phosphorylated state, to transfer potassium inside the cell"[35] After this essential binding occurs, a dephosphorylation of the alpha subunit occurs which reduces the effect of cardiac disease. It is via the inhibiting of the Na+
/K+
-ATPase that sodium levels will begin to increase within the cell which ultimately increases the concentration of intracellular calcium via the sodium-calcium exchanger. This increased presence of calcium is what allows for the force of contraction to be increased. In the case of patients where the heart is not pumping hard enough to provide what is needed for the body, use of digoxin helps to temporarily overcome this.
Discovery
Na+
/K+
-ATPase was proposed by Jens Christian Skou in 1957 while working as assistant professor at the Department of Physiology, University of Aarhus, Denmark . He published his work that year.[36]
In 1997, he received one-half of the Nobel Prize in Chemistry "for the first discovery of an ion-transporting enzyme, Na+
,K+
-ATPase."[37]
Genes
- Alpha: ATP1A1Template:Gene2, ATP1A2Template:Gene2, ATP1A3Template:Gene2, ATP1A4Template:Gene2. ATP1A1 is expressed ubiquitously in vertebrates, and ATP1A3 in neural tissue. ATP1A2 is also known as "alpha(+)". ATP1A4 is specific to mammals.
- Beta: ATP1B1Template:Gene2, Template:Gene2, ATP1B3Template:Gene2, Template:Gene2
The parallel evolution of resistance to cardiotonic steroids in many vertebrates
Several studies have detailed the evolution of cardiotonic steroid resistance of the alpha-subunit gene family of Na/K-ATPase (ATP1A) in vertebrates via amino acid substitutions most often located in the first extracellular loop domain.[38][39][40][41][42][43][44] Amino acid substitutions conferring cardiotonic steroid resistance have evolved independently many times in all major groups of tetrapods.[42] ATP1A1 has been duplicated in some groups of frogs and neofunctionlised duplicates carry the same cardiotonic steroid resistance substitutions (Q111R and N122D) found in mice, rats and other muroids.[45][38][39][40]
In insects
In Drosophila melanogaster, the alpha-subunit of Na+
/K+
-ATPase has two paralogs, ATPα (ATPα1) and JYalpha (ATPα2), resulting from an ancient duplication in insects.[46] In Drosophila, ATPα1 is ubiquitously and highly expressed, whereas ATPα2 is most highly expressed in male testes and is essential for male fertility. Insects have at least one copy of both genes, and occasionally duplications. Low expression of ATPα2 has also been noted in other insects. Duplications and neofunctionalization of ATPα1 have been observed in insects that are adapted to cardiotonic steroid toxins such as cardenolides and bufadienolides.[46][47][48][49][50] Insects adapted to cardiotonic steroids typically have a number of amino acid substitutions, most often in the first extra-cellular loop of ATPα1, that confer resistance to cardiotonic steroid inhibition.[51][52]
See also
- Sodium-calcium exchanger
- Thyroid hormone
- V-ATPase
References
- ↑ 1.0 1.1 "Sodium Transporters in Human Health and Disease (Figure 2)". Frontiers in Physiology 11: 588664. 2021. doi:10.3389/fphys.2020.588664. PMID 33716756.
- ↑ "The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease". Frontiers in Physiology 8: 371. June 2017. doi:10.3389/fphys.2017.00371. PMID 28634454.
- ↑ 3.0 3.1 Textbook of medical physiology. St. Louis, Mo: Elsevier Saunders. 2006. ISBN 978-0-7216-0240-0.
- ↑ "Section 20-3: ATP-Driven Active Transport". Biochemistry (4th ed.). John Wiley & Sons. December 2010. p. 759. ISBN 978-0-470-57095-1.
- ↑ "Updated energy budgets for neural computation in the neocortex and cerebellum". Journal of Cerebral Blood Flow and Metabolism 32 (7): 1222–32. July 2012. doi:10.1038/jcbfm.2012.35. PMID 22434069.
- ↑ "Transepithelial transport in cell culture: bioenergetics of Na-, D-glucose-coupled transport". Journal of Cellular Physiology 114 (3): 263–6. March 1983. doi:10.1002/jcp.1041140303. PMID 6833401.
- ↑ "Compartmentation of carbohydrate metabolism in vascular smooth muscle". The American Journal of Physiology 252 (3 Pt 1): C328-34. March 1987. doi:10.1152/ajpcell.1987.252.3.c328. PMID 3030131.
- ↑ "The Na+/K+ pump of cardiac Purkinje cells is preferentially fuelled by glycolytic ATP production". Pflügers Archiv 422 (4): 380–5. January 1993. doi:10.1007/bf00374294. PMID 8382364.
- ↑ "Na+-K+ pumps in the transverse tubular system of skeletal muscle fibers preferentially use ATP from glycolysis". American Journal of Physiology. Cell Physiology 293 (3): C967-77. September 2007. doi:10.1152/ajpcell.00132.2007. PMID 17553934.
- ↑ "Effects of reduced muscle glycogen on excitation-contraction coupling in rat fast-twitch muscle: a glycogen removal study". Journal of Muscle Research and Cell Motility 40 (3–4): 353–364. December 2019. doi:10.1007/s10974-019-09524-y. PMID 31236763.
- ↑ "Inhibition of glycogenolysis prolongs action potential repriming period and impairs muscle function in rat skeletal muscle". The Journal of Physiology 598 (4): 789–803. February 2020. doi:10.1113/JP278543. PMID 31823376.
- ↑ "The Na/K pump, Cl ion, and osmotic stabilization of cells". Proceedings of the National Academy of Sciences of the United States of America 100 (10): 6257–62. May 2003. doi:10.1073/pnas.0931278100. PMID 12730376. Bibcode: 2003PNAS..100.6257A.
- ↑ 13.0 13.1 13.2 "Suppression of Na+/K+-ATPase activity during estivation in the land snail Otala lactea". The Journal of Experimental Biology 209 (Pt 4): 677–88. February 2006. doi:10.1242/jeb.02052. PMID 16449562.
- ↑ "Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex". Molecular Biology of the Cell 16 (9): 4034–45. September 2005. doi:10.1091/mbc.E05-04-0295. PMID 15975899.
- ↑ "Binding of Src to Na+/K+-ATPase forms a functional signaling complex". Molecular Biology of the Cell 17 (1): 317–26. January 2006. doi:10.1091/mbc.E05-08-0735. PMID 16267270.
- ↑ "NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells". The Journal of Biological Chemistry 284 (31): 21066–76. July 2009. doi:10.1074/jbc.M109.013821. PMID 19506077.
- ↑ "Interaction of the alpha subunit of Na,K-ATPase with cofilin". The Biochemical Journal 353 (Pt 2): 377–85. January 2001. doi:10.1042/0264-6021:3530377. PMID 11139403.
- ↑ "The sodium-potassium pump controls the intrinsic firing of the cerebellar Purkinje neuron". PLOS ONE 7 (12): e51169. December 2012. doi:10.1371/journal.pone.0051169. PMID 23284664. Bibcode: 2012PLoSO...751169F.
- ↑ "Prolonged Intracellular Na+ Dynamics Govern Electrical Activity in Accessory Olfactory Bulb Mitral Cells". PLOS Biology 13 (12): e1002319. December 2015. doi:10.1371/journal.pbio.1002319. PMID 26674618.
- ↑ "The Slow Dynamics of Intracellular Sodium Concentration Increase the Time Window of Neuronal Integration: A Simulation Study" (in English). Frontiers in Computational Neuroscience 11: 85. 2017. doi:10.3389/fncom.2017.00085. PMID 28970791.
- ↑ "The sodium-potassium pump is an information processing element in brain computation". Frontiers in Physiology 5 (472): 472. December 2014. doi:10.3389/fphys.2014.00472. PMID 25566080.
- ↑ "Paying the price at the pump: dystonia from mutations in a Na+/K+-ATPase". Neuron 43 (2): 153–4. July 2004. doi:10.1016/j.neuron.2004.07.002. PMID 15260948.
- ↑ "The neural substrates of rapid-onset Dystonia-Parkinsonism". Nature Neuroscience 14 (3): 357–65. March 2011. doi:10.1038/nn.2753. PMID 21297628.
- ↑ "Simulation of alcohol action upon a detailed Purkinje neuron model and a simpler surrogate model that runs >400 times faster". BMC Neuroscience 16 (27): 27. April 2015. doi:10.1186/s12868-015-0162-6. PMID 25928094.
- ↑ "The Neuroscience Reason We Fall Over When Drunk". 4 April 2015. http://www.science20.com/michael_forrest/the_neuroscience_reason_we_fall_over_when_drunk-155301.
- ↑ "Imaging correlates of decreased axonal Na+/K+ ATPase in chronic multiple sclerosis lesions". Annals of Neurology 63 (4): 428–35. April 2008. doi:10.1002/ana.21381. PMID 18438950.
- ↑ "Early role for a Na+,K+-ATPase (ATP1A3) in brain development". Proceedings of the National Academy of Sciences of the United States of America 118 (25): e2023333118. June 2021. doi:10.1073/pnas.2023333118. PMID 34161264. Bibcode: 2021PNAS..11823333S.
- ↑ "The inositol pyrophosphate 5-InsP7 drives sodium-potassium pump degradation by relieving an autoinhibitory domain of PI3K p85α". Science Advances 6 (44): eabb8542. October 2020. doi:10.1126/sciadv.abb8542. PMID 33115740. Bibcode: 2020SciA....6.8542C.
- ↑ "Thyroid hormone upregulates Na,K-ATPase α and β mRNA in primary cultures of proximal tubule cells". Life Sciences 60 (6): 375–382. January 1997. doi:10.1016/S0024-3205(96)00661-3. PMID 9031683.
- ↑ "Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis". The American Journal of Physiology 232 (5): C165-73. May 1977. doi:10.1152/ajpcell.1977.232.5.C165. PMID 324293.
- ↑ "Role of endogenous cardiotonic steroids in sodium homeostasis". Nephrology, Dialysis, Transplantation 23 (9): 2723–9. September 2008. doi:10.1093/ndt/gfn325. PMID 18556748.
- ↑ "Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na+ pump, the Na+/Ca2+ exchanger and TRPC proteins". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1802 (12): 1219–29. December 2010. doi:10.1016/j.bbadis.2010.02.011. PMID 20211726.
- ↑ "On the differences between ouabain and digitalis glycosides". American Journal of Therapeutics 21 (1): 35–42. 2014. doi:10.1097/MJT.0b013e318217a609. PMID 21642827.
- ↑ "The role of cardiotonic steroids in the pathogenesis of cardiomyopathy in chronic kidney disease". Nephron Clinical Practice 128 (1–2): 11–21. 2014. doi:10.1159/000363301. PMID 25341357.
- ↑ "Na+/K+-ATPase and inhibitors (Digoxin)". Pharmacorama. https://www.pharmacorama.com/en/Sections/NAK-ATPase-Digoxin.php.
- ↑ "The influence of some cations on an adenosine triphosphatase from peripheral nerves". Biochimica et Biophysica Acta 23 (2): 394–401. February 1957. doi:10.1016/0006-3002(57)90343-8. PMID 13412736.
- ↑ "The Nobel Prize in Chemistry 1997". NobelPrize.org. Nobel Media AB. 15 October 1997. https://www.nobelprize.org/prizes/chemistry/1997/summary/.
- ↑ 38.0 38.1 Moore, David J.; Halliday, Damien C. T.; Rowell, David M.; Robinson, Anthony J.; Keogh, J. Scott (2009-08-23). "Positive Darwinian selection results in resistance to cardioactive toxins in true toads (Anura: Bufonidae)". Biology Letters 5 (4): 513–516. doi:10.1098/rsbl.2009.0281. ISSN 1744-9561. PMID 19465576.
- ↑ 39.0 39.1 Hernández Poveda M (2022) Convergent evolution of neo-functionalized duplications of ATP1A1 in dendrobatid and grass frogs. MS Thesis Dissertation. Universidad de los Andes
- ↑ 40.0 40.1 Mohammadi, Shabnam; Yang, Lu; Harpak, Arbel; Herrera-Álvarez, Santiago; Rodríguez-Ordoñez, María del Pilar; Peng, Julie; Zhang, Karen; Storz, Jay F. et al. (2021-06-21). "Concerted evolution reveals co-adapted amino acid substitutions in frogs that prey on toxic toads". Current Biology 31 (12): 2530–2538.e10. doi:10.1016/j.cub.2021.03.089. ISSN 0960-9822. PMID 33887183.
- ↑ Mohammadi, Shabnam; Brodie, Edmund D.; Neuman-Lee, Lorin A.; Savitzky, Alan H. (2016-05-01). "Mutations to the cardiotonic steroid binding site of Na+/K+-ATPase are associated with high level of resistance to gamabufotalin in a natricine snake". Toxicon 114: 13–15. doi:10.1016/j.toxicon.2016.02.019. ISSN 0041-0101. https://www.sciencedirect.com/science/article/pii/S0041010116300368.
- ↑ 42.0 42.1 Mohammadi, Shabnam; Herrera-Álvarez, Santiago; Yang, Lu; Rodríguez-Ordoñez, María del Pilar; Zhang, Karen; Storz, Jay F.; Dobler, Susanne; Crawford, Andrew J. et al. (2022-08-16). "Constraints on the evolution of toxin-resistant Na,K-ATPases have limited dependence on sequence divergence". PLOS Genetics 18 (8): e1010323. doi:10.1371/journal.pgen.1010323. ISSN 1553-7390. PMID 35972957.
- ↑ Mohammadi, Shabnam; Özdemir, Halil İbrahim; Ozbek, Pemra; Sumbul, Fidan; Stiller, Josefin; Deng, Yuan; Crawford, Andrew J; Rowland, Hannah M et al. (2022-12-06). "Epistatic Effects Between Amino Acid Insertions and Substitutions Mediate Toxin resistance of Vertebrate Na+,K+-ATPases". Molecular Biology and Evolution 39 (12): msac258. doi:10.1093/molbev/msac258. ISSN 0737-4038. PMID 36472530.
- ↑ Ujvari, Beata; Mun, Hee-chang; Conigrave, Arthur D.; Bray, Alessandra; Osterkamp, Jens; Halling, Petter; Madsen, Thomas (January 2013). "ISOLATION BREEDS NAIVETY: ISLAND LIVING ROBS AUSTRALIAN VARANID LIZARDS OF TOAD-TOXIN IMMUNITY VIA FOUR-BASE-PAIR MUTATION" (in en). Evolution 67 (1): 289–294. doi:10.1111/j.1558-5646.2012.01751.x. https://academic.oup.com/evolut/article/67/1/289/6851464.
- ↑ Price, Elmer M.; Lingrel, Jerry B. (1988-11-01). "Structure-function relationships in the sodium-potassium ATPase .alpha. subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme" (in en). Biochemistry 27 (22): 8400–8408. doi:10.1021/bi00422a016. ISSN 0006-2960. https://pubs.acs.org/doi/abs/10.1021/bi00422a016.
- ↑ 46.0 46.1 Zhen, Ying; Aardema, Matthew L.; Medina, Edgar M.; Schumer, Molly; Andolfatto, Peter (2012-09-28). "Parallel Molecular Evolution in an Herbivore Community" (in en). Science 337 (6102): 1634–1637. doi:10.1126/science.1226630. ISSN 0036-8075. PMID 23019645. Bibcode: 2012Sci...337.1634Z.
- ↑ Yang, L.; Ravikanthachari, N.; Mariño-Pérez, R.; Deshmukh, R.; Wu, M.; Rosenstein, A.; Kunte, K.; Song, H. et al. (2019). "Predictability in the evolution of Orthopteran cardenolide insensitivity". Philosophical Transactions of the Royal Society of London, Series B 374 (1777): 20180246. doi:10.1098/rstb.2018.0246. PMID 31154978.
- ↑ Petschenka Georg, Vera Wagschal, Michael von Tschirnhaus, Alexander Donath, Susanne Dobler 2017 Petschenka, G.; Wagschal, V.; von Tschirnhaus, M.; Donath, A.; Dobler, S. (2017). "Convergently Evolved Toxic Secondary Metabolites in Plants Drive the Parallel Molecular Evolution of Insect Resistance". The American Naturalist 190 (S1): S29–S43. doi:10.1086/691711. PMID 28731826. https://pubmed.ncbi.nlm.nih.gov/28731826/.
- ↑ Dobler Susanne, Vera Wagschal, Niels Pietsch, Nadja Dahdouli, Fee Meinzer, Renja Romey-Glüsing, Kai Schütte. 2019 Yang, L.; Borne, F.; Betz, A.; Aardema, M. L.; Zhen, Y.; Peng, J.; Visconti, R.; Wu, M. et al. (2023). "New ways to acquire resistance: imperfect convergence in insect adaptations to a potent plant toxin". bioRxiv : The Preprint Server for Biology. doi:10.1101/2023.03.08.531760. PMID 36945443.
- ↑ Lu Yang, Flora Borne, Matthew L Aardema, Ying Zhen, Julie Peng, Mariana Wu, Regina Visconti, Anja Betz, Bartholomew P Roland, Aaron D Talsma, Mike J Palladino, Georg Petschenka, Peter Andolfatto. 2023 Yang, Lu; Borne, Flora; Betz, Anja; Aardema, Matthew L.; Zhen, Ying; Peng, Julie; Visconti, Regina; Wu, Mariana et al. (2023). "The path to "femmes fatales": the evolution of toxin resistance in predatory fireflies". bioRxiv : The Preprint Server for Biology. doi:10.1101/2023.03.08.531760. PMID 36945443.
- ↑ "Molecular adaptation of Chrysochus leaf beetles to toxic compounds in their food plants". Molecular Biology and Evolution 21 (2): 218–21. 2004. doi:10.1093/molbev/msg240. PMID 12949136. https://academic.oup.com/mbe/article/21/2/218/1187788.
- ↑ Dobler, S., Dalla, S., Wagschal, V., & Agrawal, A. A. (2012). Community-wide convergent evolution in insect adaptation to toxic cardenolides by substitutions in the Na,K-ATPase. Proceedings of the National Academy of Sciences, 109(32), 13040–13045. https://doi.org/10.1073/pnas.1202111109
External links
- Sodium,+Potassium+ATPase at the US National Library of Medicine Medical Subject Headings (MeSH)
- RCSB Protein Data Bank: Sodium–Potassium Pump
- A video by Khan Academy.
 |