Biology:EF-Tu
| Elongation Factor Thermo Unstable | |||||||||
|---|---|---|---|---|---|---|---|---|---|
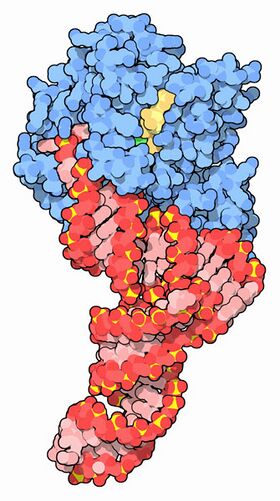 EF-Tu (blue) complexed with tRNA (red) and GTP (yellow) [1] | |||||||||
| Identifiers | |||||||||
| Symbol | EF-Tu | ||||||||
| Pfam | GTP_EFTU | ||||||||
| Pfam clan | CL0023 | ||||||||
| InterPro | IPR004541 | ||||||||
| PROSITE | PDOC00273 | ||||||||
| CATH | 1ETU | ||||||||
| SCOP2 | 1ETU / SCOPe / SUPFAM | ||||||||
| CDD | cd00881 | ||||||||
| |||||||||
| EF-Tu | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | GTP_EFTU_D2 | ||||||||
| Pfam | PF03144 | ||||||||
| InterPro | IPR004161 | ||||||||
| CDD | cd01342 | ||||||||
| |||||||||
| Elongation factor Tu domain 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | GTP_EFTU_D3 | ||||||||
| Pfam | PF03143 | ||||||||
| InterPro | IPR004160 | ||||||||
| CDD | cd01513 | ||||||||
| |||||||||
EF-Tu (elongation factor thermo unstable) is a prokaryotic elongation factor responsible for catalyzing the binding of an aminoacyl-tRNA (aa-tRNA) to the ribosome. It is a G-protein, and facilitates the selection and binding of an aa-tRNA to the A-site of the ribosome. As a reflection of its crucial role in translation, EF-Tu is one of the most abundant and highly conserved proteins in prokaryotes.[2][3][4] It is found in eukaryotic mitochondria as TUFM.[5]
As a family of elongation factors, EF-Tu also includes its eukaryotic and archaeal homolog, the alpha subunit of eEF-1 (EF-1A).
Background
Elongation factors are part of the mechanism that synthesizes new proteins through translation in the ribosome. Transfer RNAs (tRNAs) carry the individual amino acids that become integrated into a protein sequence, and have an anticodon for the specific amino acid that they are charged with. Messenger RNA (mRNA) carries the genetic information that encodes the primary structure of a protein, and contains codons that code for each amino acid. The ribosome creates the protein chain by following the mRNA code and integrating the amino acid of an aminoacyl-tRNA (also known as a charged tRNA) to the growing polypeptide chain.[6][7]
There are three sites on the ribosome for tRNA binding. These are the aminoacyl/acceptor site (abbreviated A), the peptidyl site (abbreviated P), and the exit site (abbreviated E). The P-site holds the tRNA connected to the polypeptide chain being synthesized, and the A-site is the binding site for a charged tRNA with an anticodon complementary to the mRNA codon associated with the site. After binding of a charged tRNA to the A-site, a peptide bond is formed between the growing polypeptide chain on the P-site tRNA and the amino acid of the A-site tRNA, and the entire polypeptide is transferred from the P-site tRNA to the A-site tRNA. Then, in a process catalyzed by the prokaryotic elongation factor EF-G (historically known as translocase), the coordinated translocation of the tRNAs and mRNA occurs, with the P-site tRNA moving to the E-site, where it dissociates from the ribosome, and the A-site tRNA moves to take its place in the P-site.[6][7]
Biological functions
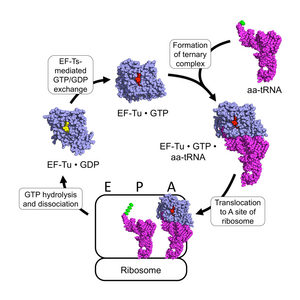
Protein synthesis
EF-Tu participates in the polypeptide elongation process of protein synthesis. In prokaryotes, the primary function of EF-Tu is to transport the correct aa-tRNA to the A-site of the ribosome. As a G-protein, it uses GTP to facilitate its function. Outside of the ribosome, EF-Tu complexed with GTP (EF-Tu • GTP) complexes with aa-tRNA to form a stable EF-Tu • GTP • aa-tRNA ternary complex.[8] EF-Tu • GTP binds all correctly-charged aa-tRNAs with approximately identical affinity, except those charged with initiation residues and selenocysteine.[9][10] This can be accomplished because although different amino acid residues have varying side-chain properties, the tRNAs associated with those residues have varying structures to compensate for differences in side-chain binding affinities.[11][12]
The binding of an aa-tRNA to EF-Tu • GTP allows for the ternary complex to be translocated to the A-site of an active ribosome, in which the anticodon of the tRNA binds to the codon of the mRNA. If the correct anticodon binds to the mRNA codon, the ribosome changes configuration and alters the geometry of the GTPase domain of EF-Tu, resulting in the hydrolysis of the GTP associated with the EF-Tu to GDP and Pi. As such, the ribosome functions as a GTPase-activating protein (GAP) for EF-Tu. Upon GTP hydrolysis, the conformation of EF-Tu changes drastically and dissociates from the aa-tRNA and ribosome complex.[4][13] The aa-tRNA then fully enters the A-site, where its amino acid is brought near the P-site's polypeptide and the ribosome catalyzes the covalent transfer of the polypeptide onto the amino acid.[10]
In the cytoplasm, the deactivated EF-Tu • GDP is acted on by the prokaryotic elongation factor EF-Ts, which causes EF-Tu to release its bound GDP. Upon dissociation of EF-Ts, EF-Tu is able to complex with a GTP due to the 5– to 10–fold higher concentration of GTP than GDP in the cytoplasm, resulting in reactivated EF-Tu • GTP, which can then associate with another aa-tRNA.[8][13]
Maintaining translational accuracy
EF-Tu contributes to translational accuracy in three ways. In translation, a fundamental problem is that near-cognate anticodons have similar binding affinity to a codon as cognate anticodons, such that anticodon-codon binding in the ribosome alone is not sufficient to maintain high translational fidelity. This is addressed by the ribosome not activating the GTPase activity of EF-Tu if the tRNA in the ribosome's A-site does not match the mRNA codon, thus preferentially increasing the likelihood for the incorrect tRNA to leave the ribosome.[14] Additionally, regardless of tRNA matching, EF-Tu also induces a delay after freeing itself from the aa-tRNA, before the aa-tRNA fully enters the A-site (a process called accommodation). This delay period is a second opportunity for incorrectly charged aa-tRNAs to move out of the A-site before the incorrect amino acid is irreversibly added to the polypeptide chain.[15][16] A third mechanism is the less well understood function of EF-Tu to crudely check aa-tRNA associations and reject complexes where the amino acid is not bound to the correct tRNA coding for it.[11]
Other functions
EF-Tu has been found in large quantities in the cytoskeletons of bacteria, co-localizing underneath the cell membrane with MreB, a cytoskeletal element that maintains cell shape.[17][18] Defects in EF-Tu have been shown to result in defects in bacterial morphology.[19] Additionally, EF-Tu has displayed some chaperone-like characteristics, with some experimental evidence suggesting that it promotes the refolding of a number of denatured proteins in vitro.[20][21]
Structure

EF-Tu is a monomeric protein with molecular weight around 43 kDa in Escherichia coli.[22][23][24] The protein consists of three structural domains: a GTP-binding domain and two oligonucleotide-binding domains, often referred to as domain 2 and domain 3. The N-terminal domain I of EF-Tu is the GTP-binding domain. It consists of a six beta-strand core flanked by six alpha-helices.[8] Domains II and III of EF-Tu, the oligonucleotide-binding domains, both adopt beta-barrel structures.[25][26]
The GTP-binding domain I undergoes a dramatic conformational change upon GTP hydrolysis to GDP, allowing EF-Tu to dissociate from aa-tRNA and leave the ribosome.[27] Reactivation of EF-Tu is achieved by GTP binding in the cytoplasm, which leads to a significant conformational change that reactivates the tRNA-binding site of EF-Tu. In particular, GTP binding to EF-Tu results in a ~90° rotation of domain I relative to domains II and III, exposing the residues of the tRNA-binding active site.[28]
Domain 2 adopts a beta-barrel structure, and is involved in binding to charged tRNA.[29] This domain is structurally related to the C-terminal domain of EF2, to which it displays weak sequence similarity. This domain is also found in other proteins such as translation initiation factor IF-2 and tetracycline-resistance proteins. Domain 3 represents the C-terminal domain, which adopts a beta-barrel structure, and is involved in binding to both charged tRNA and to EF1B (or EF-Ts).[30]
Evolution
The GTP-binding domain is conserved in both EF-1alpha/EF-Tu and also in EF-2/EF-G and thus seems typical for GTP-dependent proteins which bind non-initiator tRNAs to the ribosome. The GTP-binding translation factor family also includes the eukaryotic peptide chain release factor GTP-binding subunits[31] and prokaryotic peptide chain release factor 3 (RF-3);[32] the prokaryotic GTP-binding protein lepA and its homologue in yeast (GUF1) and Caenorhabditis elegans (ZK1236.1); yeast HBS1;[33] rat Eef1a1 (formerly "statin S1");[34] and the prokaryotic selenocysteine-specific elongation factor selB.[35]
Disease relevance
Along with the ribosome, EF-Tu is one of the most important targets for antibiotic-mediated inhibition of translation.[8] Antibiotics targeting EF-Tu can be categorized into one of two groups, depending on the mechanism of action, and one of four structural families. The first group includes the antibiotics pulvomycin and GE2270A, and inhibits the formation of the ternary complex.[36] The second group includes the antibiotics kirromycin and enacyloxin, and prevents the release of EF-Tu from the ribosome after GTP hydrolysis.[37][38][39]
See also
- Prokaryotic elongation factors
- EF-Ts (elongation factor thermo stable)
- EF-G (elongation factor G)
- EF-P (elongation factor P)
- eEF-1
- EFR (EF-Tu receptor)
References
- ↑ PDB Molecule of the Month EF-Tu
- ↑ "Elongation factor Tu: a molecular switch in protein biosynthesis". Molecular Microbiology 6 (6): 683–8. March 1992. doi:10.1111/j.1365-2958.1992.tb01516.x. PMID 1573997.
- ↑ "TIGR00485: EF-Tu". March 3, 2017. https://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?uid=TIGR00485.
- ↑ 4.0 4.1 "EF-G and EF4: translocation and back-translocation on the bacterial ribosome". Nature Reviews. Microbiology 12 (2): 89–100. February 2014. doi:10.1038/nrmicro3176. PMID 24362468.
- ↑ "The human mitochondrial elongation factor tu (EF-Tu) gene: cDNA sequence, genomic localization, genomic structure, and identification of a pseudogene". Gene 197 (1–2): 325–36. Nov 1997. doi:10.1016/S0378-1119(97)00279-5. PMID 9332382.
- ↑ 6.0 6.1 "Initiation of protein synthesis in bacteria". Microbiology and Molecular Biology Reviews 69 (1): 101–23. March 2005. doi:10.1128/MMBR.69.1.101-123.2005. PMID 15755955.
- ↑ 7.0 7.1 "Ribosome structure and the mechanism of translation". Cell 108 (4): 557–72. February 2002. doi:10.1016/s0092-8674(02)00619-0. PMID 11909526.
- ↑ 8.0 8.1 8.2 8.3 Mechanisms of EF-Tu, a pioneer GTPase. 71. 2002-01-01. 513–51. doi:10.1016/S0079-6603(02)71050-7. ISBN 9780125400718.
- ↑ "Translation elongation factor EFTu/EF1A, bacterial/organelle (IPR004541)". http://www.ebi.ac.uk/interpro/entry/IPR004541.
- ↑ 10.0 10.1 Diwan, Joyce (2008). "Translation: Protein Synthesis". https://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb2/part1/translate.htm.
- ↑ 11.0 11.1 "Uniform binding of aminoacyl-tRNAs to elongation factor Tu by thermodynamic compensation". Science 294 (5540): 165–8. October 2001. doi:10.1126/science.1064242. PMID 11588263. Bibcode: 2001Sci...294..165L.
- ↑ "Relative affinities of all Escherichia coli aminoacyl-tRNAs for elongation factor Tu-GTP". The Journal of Biological Chemistry 259 (8): 5010–6. April 1984. doi:10.1016/S0021-9258(17)42947-4. PMID 6370998.
- ↑ 13.0 13.1 "The ternary complex of EF-Tu and its role in protein biosynthesis". Current Opinion in Structural Biology 7 (1): 110–6. February 1997. doi:10.1016/s0959-440x(97)80014-0. PMID 9032056.
- ↑ "Elongation factors on the ribosome". Current Opinion in Structural Biology 15 (3): 349–54. June 2005. doi:10.1016/j.sbi.2005.05.004. PMID 15922593.
- ↑ "Accommodation of aminoacyl-tRNA into the ribosome involves reversible excursions along multiple pathways". RNA 16 (6): 1196–204. June 2010. doi:10.1261/rna.2035410. PMID 20427512.
- ↑ "How EF-Tu can contribute to efficient proofreading of aa-tRNA by the ribosome". Nature Communications 7: 13314. October 2016. doi:10.1038/ncomms13314. PMID 27796304. Bibcode: 2016NatCo...713314N.
- ↑ "Bacterial translation elongation factor EF-Tu interacts and colocalizes with actin-like MreB protein". Proceedings of the National Academy of Sciences of the United States of America 107 (7): 3163–8. February 2010. doi:10.1073/pnas.0911979107. PMID 20133608. Bibcode: 2010PNAS..107.3163D.
- ↑ "Cytoskeletons in prokaryotes". Cell Biology International 27 (5): 429–38. 2003-01-01. doi:10.1016/s1065-6995(03)00035-0. PMID 12758091.
- ↑ "Cytoskeletal elements in bacteria Mycoplasma pneumoniae, Thermoanaerobacterium sp., and Escherichia coli as revealed by electron microscopy". Journal of Molecular Microbiology and Biotechnology 11 (3–5): 228–43. 2006-01-01. doi:10.1159/000094057. PMID 16983198.
- ↑ "Protein-disulfide isomerase activity of elongation factor EF-Tu". Biochemical and Biophysical Research Communications 252 (1): 156–61. November 1998. doi:10.1006/bbrc.1998.9591. PMID 9813162.
- ↑ "Renaturation of rhodanese by translational elongation factor (EF) Tu. Protein refolding by EF-Tu flexing". The Journal of Biological Chemistry 272 (51): 32206–10. December 1997. doi:10.1074/jbc.272.51.32206. PMID 9405422.
- ↑ "Purification of elongation factors EF-Tu and EF-G from Escherichia coli by covalent chromatography on thiol-sepharose". Protein Expression and Purification 14 (1): 65–70. October 1998. doi:10.1006/prep.1998.0922. PMID 9758752.
- ↑ "Mapping Escherichia coli elongation factor Tu residues involved in binding of aminoacyl-tRNA". The Journal of Biological Chemistry 271 (34): 20406–11. August 1996. doi:10.1074/jbc.271.34.20406. PMID 8702777.
- ↑ "Isolation of the protein synthesis elongation factors EF-Tu, EF-Ts, and EF-G from Escherichia coli". Nucleic Acids and Protein Synthesis Part H. Methods in Enzymology. 60. 1979-01-01. pp. 593–606. doi:10.1016/s0076-6879(79)60056-3. ISBN 9780121819606. https://archive.org/details/nucleicacids0000unse/page/593.
- ↑ "Crystal structure of the EF-Tu.EF-Ts complex from Thermus thermophilus". Nature Structural Biology 4 (8): 650–6. August 1997. doi:10.1038/nsb0897-650. PMID 9253415.
- ↑ "Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog". Science 270 (5241): 1464–72. December 1995. doi:10.1126/science.270.5241.1464. PMID 7491491.
- ↑ "A conserved amino acid sequence around Arg-68 of Artemia elongation factor 1 alpha is involved in the binding of guanine nucleotides and aminoacyl transfer RNAs". Biochimie 69 (9): 983–9. September 1987. doi:10.1016/0300-9084(87)90232-x. PMID 3126836.
- ↑ "The crystal structure of elongation factor EF-Tu from Thermus aquaticus in the GTP conformation". Structure 1 (1): 35–50. September 1993. doi:10.1016/0969-2126(93)90007-4. PMID 8069622.
- ↑ "Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog". Science 270 (5241): 1464–72. December 1995. doi:10.1126/science.270.5241.1464. PMID 7491491.
- ↑ "Crystal structure of the EF-Tu.EF-Ts complex from Thermus thermophilus". Nat. Struct. Biol. 4 (8): 650–6. August 1997. doi:10.1038/nsb0897-650. PMID 9253415.
- ↑ "The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae". EMBO J. 14 (17): 4365–73. September 1995. doi:10.1002/j.1460-2075.1995.tb00111.x. PMID 7556078.
- ↑ "Function of polypeptide chain release factor RF-3 in Escherichia coli. RF-3 action in termination is predominantly at UGA-containing stop signals". J. Biol. Chem. 270 (18): 10595–600. May 1995. doi:10.1074/jbc.270.18.10595. PMID 7737996.
- ↑ "The translation machinery and 70 kd heat shock protein cooperate in protein synthesis". Cell 71 (1): 97–105. October 1992. doi:10.1016/0092-8674(92)90269-I. PMID 1394434.
- ↑ "Isolation and characterization of the rat chromosomal gene for a polypeptide (pS1) antigenically related to statin". J. Biol. Chem. 266 (16): 10429–37. June 1991. doi:10.1016/S0021-9258(18)99243-4. PMID 1709933.
- ↑ "Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein". Nature 342 (6248): 453–6. November 1989. doi:10.1038/342453a0. PMID 2531290. Bibcode: 1989Natur.342..453F.
- ↑ "Antibiotic GE2270 a: a novel inhibitor of bacterial protein synthesis. I. Isolation and characterization". The Journal of Antibiotics 44 (7): 693–701. July 1991. doi:10.7164/antibiotics.44.693. PMID 1908853.
- ↑ "Inhibitory mechanisms of antibiotics targeting elongation factor Tu". Current Protein & Peptide Science 3 (1): 121–31. February 2002. doi:10.2174/1389203023380855. PMID 12370016.
- ↑ "Elongation factors in protein biosynthesis". Trends in Biochemical Sciences 28 (8): 434–41. August 2003. doi:10.1016/S0968-0004(03)00162-2. PMID 12932732.
- ↑ "Elongation factor Tu-targeted antibiotics: four different structures, two mechanisms of action". FEBS Letters 580 (19): 4576–81. August 2006. doi:10.1016/j.febslet.2006.07.039. PMID 16876786.
External links
- Peptide+Elongation+Factor+Tu at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P49410 (Elongation factor Tu, mitochondrial) at the PDBe-KB.
 |
