Chemistry:Isoxanthohumol
From HandWiki
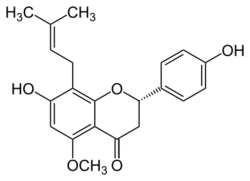
| |
| Names | |
|---|---|
| IUPAC name
7-hydroxy-2-(4-hydroxyphenyl)-5-methoxy-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C21H22O5 | |
| Molar mass | 354.402 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Isoxanthohumol is a prenylflavonoid, and it is a phytoestrogen. It is abbreviated as IX or IXN.
8-Prenylnaringenin can be produced from isoxanthohumol by flora in the human intestine,[1] and by fungi in cell cultures.[2]
This prenylflavonoid is found in hops and beer. It has limited estrogenic activity. At the concentration found in beer, it is unlikely to have an estrogenic effect in breast tissue.[3]
Derivatives of isoxanthohumol are: 7,4′-Di-O-methylisoxanthohumol; 7-O-methylisoxanthohumol; 7-O-n-pentylisoxanthohumol; 7,4′-di-O-n-pentyl-8-isoxanthohumol; 7,4′-Di-O-allylisoxanthohumol; 7,4′-Di-O-acetylisoxanthohumol; and 7,4′-Di-O-palmitoylisoxanthohumol.[4]
See also
- Xanthohumol, the corresponding prenylated chalcone
References
- ↑ "The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine". Journal of Nutrition 136 (7): 1862–7. 2006. doi:10.1093/jn/136.7.1862. PMID 16772450. http://jn.nutrition.org/content/136/7/1862.long.
- ↑ Fu, Ming-Liang; Wang, Wei; Chen, Feng; Dong, Ya-Chen; Liu, Xiao-jie; Ni, Hui; Chen, Qi-he (2011). "Production of 8-Prenylnaringenin from Isoxanthohumol through Biotransformation by Fungi Cells". Journal of Agricultural and Food Chemistry 59 (13): 7419–26. doi:10.1021/jf2011722. PMID 21634799.
- ↑ Bolca, Selin; Li, Jinghu; Nikolic, Dejan; Roche, Nathalie; Blondeel, Phillip; Possemiers, Sam; De Keukeleire, Denis; Bracke, Marc et al. (2010). "Disposition of hop prenylflavonoids in human breast tissue". Molecular Nutrition & Food Research 54: S284–94. doi:10.1002/mnfr.200900519. PMID 20486208.
- ↑ "Antiproliferative activity and synthesis of 8-prenylnaringenin derivatives by demethylation of 7-O- and 4'-O-substituted isoxanthohumols". Med Chem Res 21 (12): 4230–4238. 2012. doi:10.1007/s00044-011-9967-8. PMID 23087590.
 |

