Chemistry:β-Zearalenol
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | beta-Zearalenol; beta-trans-Zearalenol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
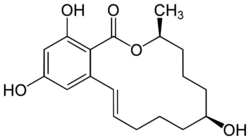
| Formula | C18H24O5 |
| Molar mass | 320.385 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
β-Zearalenol is a nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in Fusarium spp.[1] It is the β epimer of α-zearalenol and along with α-zearalenol is a major metabolite of zearalenone formed mainly in the liver but also to a lesser extent in the intestines during first-pass metabolism.[2][3] A relatively high proportion of α-zearalenol is formed from zearalenone compared to β-zearalenol in humans.[3] β-Zearalenol is about the same or slightly less potent as an estrogen relative to zearalenone.[1]
See also
- Taleranol (β-zearalanol)
- Zeranol (α-zearalanol)
- Zearalanone
References
- ↑ 1.0 1.1 "Fusarium species and their mycotoxins in infected cereals in the field and in stored grains". Fusarium: Mycotoxins, Taxonomy, Pathogenicity. Elsevier Science. January 1989. pp. 85–119. ISBN 978-1-4832-9785-9. https://books.google.com/books?id=_1KeBQAAQBAJ&pg=PA85.
- ↑ "Zearalenone". Mycotoxins in Food: Detection and Control. Woodhead Publishing. January 2004. pp. 353–366. ISBN 978-1-85573-733-4. https://books.google.com/books?id=CZ3iEhPeejoC&pg=PA356.
- ↑ 3.0 3.1 "Zearalenone". Fusarium Toxins in Cereals: A Risk Assessment. Nordic Council of Ministers. 1998. pp. 61–. ISBN 978-92-893-0149-7. https://books.google.com/books?id=jU5Wx8LkZHUC&pg=PA61.
 |

