Chemistry:Tetraxenonogold(II)
| |
| Names | |
|---|---|
| IUPAC name
Tetraxenonogold(II)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| AuXe2+4 | |
| Molar mass | 722.138 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
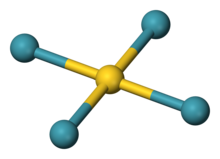
Tetraxenonogold(II), gold tetraxenide(II) or AuXe2+4 is a cationic complex with a square planar configuration of atoms. It is found in the compound AuXe2+4(Sb2F−11)2 (tetraxenonogold(II) undecafluorodiantimonate), which exists in triclinic and tetragonal crystal modifications.[1] The AuXe2+4 ion is stabilised by interactions with the fluoride atoms of the counterion. The Au−Xe bond length is 274 pm (2.74 Å).[2][3] Tetraxenonogold(II) is unusual in that it is a coordination complex of xenon, which is weakly basic. It is also unusual in that it contains gold in the +2 oxidation state. It can be produced by reduction of AuF3 by xenon in the presence of fluoroantimonic acid. The salt crystallises at low temperature.[4] Four xenon atoms bond with the gold(II) ion to make this complex.
It was the first description of a compound between a noble gas and a noble metal. It was first described in 2000 by Konrad Seppelt and Stefan Seidel. Several related compounds containing gold(III)–xenon and gold(I)–xenon bonds have since been isolated. A compound containing a mercury–xenon bond [HgXe]2+[Sb2F11]–[SbF6]– (xenonomercury(II) undecafluorodiantimonate hexafluoroantimonate) has also been isolated.[5]
References
- ↑ Wai-Kee Li; Gong-Du Zhou; Thomas C. W. Mak (2008). Advanced Structural Inorganic Chemistry. Oxford University Press. p. 678. ISBN 978-0-19-921694-9. https://archive.org/details/advancedstructur00liwa.
- ↑ Li, Wai-Kee; Zhou, Gong-Du (2008). Advanced Structural Inorganic Chemistry. Thomas C. W. Mak. Oxford University Press. p. 74. ISBN 978-0-19-921694-9. https://archive.org/details/advancedstructur00liwa.
- ↑ Mackay, Kenneth Malcolm; Mackay, Rosemary Ann; Henderson, W. (2002). Introduction to modern inorganic chemistry (6th ed.). CRC Press. p. 496. ISBN 0-7487-6420-8.
- ↑ Konrad Seppelt, Stefan Seidel; Seppelt, K (2000-10-06). "Xenon as a Complex Ligand: The Tetraxenonogold(II) Cation in AuXe2+4(Sb2F−11)2". Science 290 (5489): 117–118. doi:10.1126/science.290.5489.117. PMID 11021792. Bibcode: 2000Sci...290..117S.
- ↑ Hwang, In‐Chul; Seidel, Stefan; Seppelt, Konrad (2003-09-22). "Gold( I ) and Mercury( II ) Xenon Complexes" (in en). Angewandte Chemie International Edition 42 (36): 4392–4395. doi:10.1002/anie.200351208. ISSN 1433-7851. https://onlinelibrary.wiley.com/doi/10.1002/anie.200351208.
 |

