Biology:EZH2
 Generic protein structure example |
Enhancer of zeste homolog 2 (EZH2) is a histone-lysine N-methyltransferase enzyme (EC 2.1.1.43) encoded by EZH2 gene, that participates in histone methylation and, ultimately, transcriptional repression.[1] EZH2 catalyzes the addition of methyl groups to histone H3 at lysine 27,[2] by using the cofactor S-adenosyl-L-methionine. Methylation activity of EZH2 facilitates heterochromatin formation thereby silences gene function.[1] Remodeling of chromosomal heterochromatin by EZH2 is also required during cell mitosis.
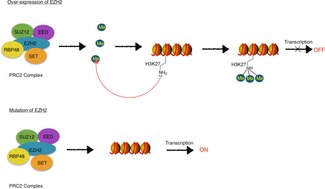
EZH2 is the functional enzymatic component of the Polycomb Repressive Complex 2 (PRC2), which is responsible for healthy embryonic development through the epigenetic maintenance of genes responsible for regulating development and differentiation.[3] EZH2 is responsible for the methylation activity of PRC2, and the complex also contains proteins required for optimal function (EED, SUZ12, JARID2, AEBP2, RbAp46/48, and PCL).[4]
Mutation or over-expression of EZH2 has been linked to many forms of cancer.[5] EZH2 inhibits genes responsible for suppressing tumor development, and blocking EZH2 activity may slow tumor growth. EZH2 has been targeted for inhibition because it is upregulated in multiple cancers including, but not limited to, breast,[6] prostate,[7] melanoma,[8] and bladder cancer.[9] Mutations in the EZH2 gene are also associated with Weaver syndrome, a rare congenital disorder,[10] and EZH2 is involved in causing neurodegenerative symptoms in the nervous system disorder, ataxia telangiectasia.[11]
Function
| Histone-lysine N-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.1.1.43 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
EZH2 is the catalytic subunit of the Polycomb Repressive Complex 2 (PRC2).[12] EZH2's catalytic activity relies on its formation of a complex with at least two other PRC2 components, SUZ12 and EED.[13]
As a histone methyltransferase (HMTase), EZH2's primary function is to methylate Lys-27 on histone 3 (H3K27me) by transferring a methyl group from the cofactor S-adenosyl-L-methionine (SAM). EZH2 is capable of mono-, di-, and tri-methylation of H3K27 and has been associated with a variety of biological functions, including transcriptional regulation in hematopoiesis, development, and cell differentiation.[13][14][15][16]
EZH2 has also been identified as capable of methylating non-histone proteins.[13][14]
Transcription repression
EZH2, as a part of PRC2, catalyzes trimethylation of H3K27 (H3K27me3), which is a histone modification that has been characterized as part of the histone code.[12][16][17][18] The histone code is the theory that chemical modifications, such as methylation, acetylation, and ubiquitination, of histone proteins play distinctive roles in epigenetic regulation of gene transcription. EZH2-mediated catalysis of H3K27me3 is associated with long term transcription repression.[12][16][17]
EZH2, as well as other Polycomb group proteins, are involved in establishing and maintaining gene repression through cell division.[13][16] This transcriptionally repressive state is thought to be due to PRC2/EZH2-EED-mediated H3K27 methylation and subsequent recruitment of PRC1 which facilitates condensation of chromatin and formation of heterochromatin.[16] Heterochromatin is tightly packed chromatin which limits the accessibility of transcription machinery to the underlying DNA, thereby suppressing transcription.[19]
During cell division, heterochromatin formation is required for proper chromosome segregation.[20] PRC2/EED-EZH2 complex may also be involved in the recruitment of DNA methyltransferases (DNMTs), which results in increased DNA methylation, another epigenetic layer of transcription repression.[12][13] Specific genes that have been identified as targets of EZH2-mediated transcriptional repression include HOXA9, HOXC8, MYT1, CDKN2A and retinoic acid target genes.[12]
Transcription activation
In cancer, EZH2 may play a role in activation of transcription, independently of PRC2.[13] In breast cancer cells, EZH2 has been demonstrated to activate NF-κB target genes, which are involved in responses to stimuli.[13] The functional role of this activity and its mechanism are still unknown.
Development and cell differentiation
EZH2 plays an essential role in development. In particular, it helps control transcriptional repression of genes that regulate cell differentiation.[13][14][16][17] In embryonic stem cells, EZH2-mediated trimethylation of H3K27me3 in regions containing developmental genes appears to be important for maintenance of normal cell differentiation.[16] H3K27me3 is also important in driving X-inactivation, the silencing of one X-chromosome in females during development.[18] During X-inactivation, it is thought that EZH2 is involved in initiating heterochromatin formation by trimethylating H3K27 and that other histone methyltransferases and histone marks may be involved in maintaining the silenced state.[21]
Further, EZH2 has been identified as an essential protein involved in development and differentiation of B-cells and T-cells.[14] H3K27me3 is involved in suppressing genes that promote differentiation, thus maintaining an undifferentiated state of B- and T-cells and playing an important role in regulating hematopoiesis.[14][22][23]
Regulation of EZH2 activity
The activity of EZH2 is regulated by the post-translational phosphorylation of threonine and serine residues on EZH2.[24] Specifically, phosphorylation of T350 has been linked to an increase in EZH2 activity while phosphorylation of T492 and S21 have been linked to a decrease in EZH2 activity.[17][24] Phosphorylation of T492 has been suggested to disrupt contacts between human EZH2 and its binding partners in the PRC2 complex, thus hindering its catalytic activity.[17]
In addition to phosphorylation, it has also been shown that PRC2/EZH2-EED activity is antagonized by transcription-activating histone marks, such as acetylation of H3K27 (H3K27ac) and methylation of H3K36 (H3K36me).[17][25]
EZH2 expression is regulated by estrogen signaling in human normal breast epithelium and human breast cancers.[26]
Enzymatic activity
EZH2 function is highly dependent upon its recruitment by the PRC2 complex. In particular, WD40-repeat protein embryonic ectoderm development (EED) and zinc finger protein suppressor of zeste 12 (SUZ12) are needed to stabilize the interaction of EZH2 with its histone substrate[27][28] Recently, two isoforms of EZH2 generated from alternative splicing have been identified in humans: EZH2α and EZH2β.[29] Both isoforms contain elements that have been identified as important for EZH2 function including the nuclear localization signal, the EED and SUZ12 binding sites as well as the conserved SET domain.[29] Most studies have thus far focused on the longer isoform EZH2α, but EZH2β, which lacks exons 4 and 8, has been shown to be active.[29] Furthermore, PRC2/EZH2β complexes act on distinct genes from that of its PRC2/EZH2α counterpart suggesting that each isoform may act to regulate a specific subset of genes.[29] Additional evidence suggests that EZH2 may also be capable of lysine methylation independent of association with PRC2, when EZH2 is highly upregulated.[13]
Lysine methylation
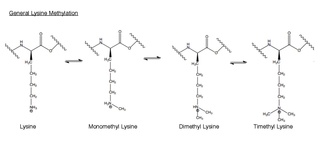
Methylation is the addition of a -CH3, or methyl group, to another molecule. In biology, methylation is typically catalyzed by enzymes, and methyl groups are commonly added to either proteins or nucleic acids. In EZH2-catalyzed methylation, the amino acid lysine in the histone h3 is methylated. This amino acid residue can be methylated up to three times on its terminal ammonium group. These methylated lysines are important in the control of mammalian gene expression and have a functional role in heterochromatin formation, X-chromosome inactivation and transcriptional regulation.[30] In mammalian chromosomes, histone lysine methylation can either activate or repress genes depending the site of methylation. Recent work has shown that at least part of the silencing function of the EZH2 complex is the methylation of histone H3 on lysine 27.[31] Methylation, and other modifications, take place on the histones. Methyl modifications can affect the binding of proteins to these histones and either activate or inhibit transcription.[20]
Mechanism of catalysis
File:EZH2 SET-domain.tiff EZH2 is a member of the SET domain family of lysine methyltransferases which function to add methyl groups to lysine side chains of substrate proteins.[32] SET methyltransferases depend on a S-Adenosyl methionine (SAM) cofactor to act as a methyl donor for their catalytic activity. SET domain proteins differ from other SAM-dependent methyltransferases in that they bind their substrate and SAM cofactor on opposite sides of the active site of the enzyme. This orientation of substrate and cofactor allows SAM to dissociate without disrupting substrate binding and can lead to multiple rounds of lysine methylation without substrate dissociation.[32]
Although neither a substrate-bound or SAM-bound crystal structure for EZH2 has been determined, STAMP structure alignment with the human SET7/9 methyltransferase shows conserved tyrosine residues in almost identical positions within the putative active site of EZH2. File:EZH2 active site residues.tiff
It had been previously suggested that tyrosine 726 in the EZH2 active site was acting as a general base to de-protonate the substrate lysine but kinetic isotope effects have indicated that active site residues are not directly involved in the chemistry of the methyltransferase reaction.[33] Instead these experiments support a mechanism in which the residues lower the pKa of the substrate lysine residue while simultaneously providing a channel for water to access the lysine side chain within the interior of the active site. Bulk solvent water can then easily deprotonate the lysine side chain, activating it for nucleophilic attack of the SAM cofactor in an SN2-like reaction resulting in transfer of the methyl group from SAM to the lysine side chain.[33]
File:Lysine Methylation by SAM.tif EZH2 primarily catalyzes mono- and di-methylation of H3K27 but a clinically relevant mutation of residue tyrosine 641 to phenylalanine (Y641F) results in higher H3K27 tri-methylation activity.[33][34] It is proposed that the removal of the hydroxyl group on Y641 abrogates steric hindrance and allows for accommodation of a third methyl group on the substrate lysine.
Clinical significance
Cancer
EZH2 is an attractive target for anti-cancer therapy because it helps cancerous cells divide and proliferate. It is found in larger amounts than in healthy cells in a wide range of cancers including breast, prostate, bladder, uterine, and renal cancers, as well as melanoma and lymphoma. EZH2 is a gene suppressor, so when it becomes overexpressed, many tumor suppressor genes that are normally turned on, are turned off. Inhibition of EZH2 function shrinks malignant tumors in some reported cases because those tumor suppressor genes are not silenced by EZH2.[35] EZH2 typically is not expressed in healthy adults; it is only found in actively dividing cells, like those active during fetal development.[36] Because of this characteristic, overexpression of EZH2 can be used as a diagnostic marker of cancer and some neurodegenerative disorders.[11] However, there are cases where it is difficult to tell whether overexpression of EZH2 is the cause of a disease, or simply a consequence. If it is only a consequence, targeting EZH2 for inhibition may not cure the disease. One example of a cancer pathway in which EZH2 plays a role is the pRB-E2F pathway. It is downstream from the pRB-E2F pathway, and signals from this pathway lead to EZH2 overexpression.[37] Another important characteristic of EZH2 is that when EZH2 is overexpressed, it can activate genes without forming PRC2. This is an issue because it means the methylation activity of the enzyme is not mediated by complex formation. In breast cancer cells, EZH2 activates genes that promote cell proliferation and survival.[13] It can also activate regulatory genes like c-myc and cyclin D1 by interacting with Wnt signaling factors.[38] Importantly, the mutation of tyrosine 641 in the active SET domain to a number of different amino acids is a common feature of some B-cell lymphomas.[39]
Inhibitors
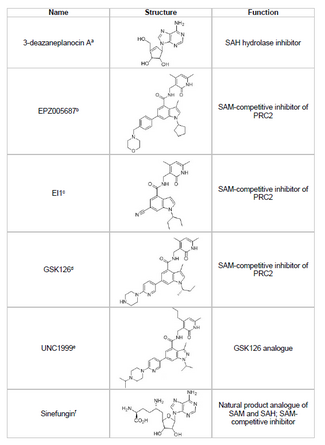
Developing an inhibitor of EZH2 and preventing unwanted histone methylation of tumor suppressor genes is a viable area of cancer research. EZH2 inhibitor development has focused on targeting the SET domain active site of the protein. Several inhibitors of EZH2 have been developed as of 2015, including 3-deazaneplanocin A (DZNep), EPZ005687, EI1, GSK126, and UNC1999.
- DZNep
- DZNep has potential antiviral and anti-cancer properties because it lowers EZH2 levels and induces apoptosis in breast and colon cancer cells.[40] DZNep inhibits the hydrolysis of S-adenosyl-L-homocysteine (SAH), which is a product-based inhibitor of all protein methyltransferases, leading to increased cellular concentrations of SAH which in turn inhibits EZH2. However, DZNep is not specific to EZH2 and also inhibits other DNA methyltransferases.
- EPZ005687
- In 2012, a company called Epizyme revealed EPZ005687, an S-adenosylmethionine (SAM) competitive inhibitor that is more selective than DZNep; it has a 50-fold increase in selectivity for EZH2 compared to EZH1. The drug blocks EZH2 activity by binding to the SET domain active site of the enzyme. EPZ005687 can also inhibit the Y641 and A677 mutants of EZH2, which may be applicable for treating non-Hodgkin's lymphoma.[41]
- Tazemetostat
- In 2013, Epizyme began Phase I clinical trials with another EZH2 inhibitor, tazemetostat (EPZ-6438), for patients with B-cell lymphoma.[45] In 2020, tazemetostat, with the tradename Tazverik, gained an FDA accelerated approval for the treatment of metastatic or locally advanced epithelioid sarcoma and an accelerated approval for the treatment of patients with relapsed follicular lymphoma later that year.[46]
- Sinefungin
- Sinefungin is another SAM-competitive inhibitor, however, like DZNep, it is not specific to EZH2.[44] It works by binding in the cofactor binding pocket of DNA methyltransferases to block methyl transfer. EI1 is another inhibitor, developed by Novartis, that showed EZH2 inhibitory activity in lymphoma tumor cells, including cells with the Y641 mutation.[42] The mechanism of this inhibitor also involves competing with the SAM cofactor for binding to EZH2.[42]
- GSK126
- GSK126 is a potent, SAM-competitive EZH2 inhibitor developed by GlaxoSmithKline, that has 150-fold selectivity over EZH1 and a Ki of 0.5-3 nM.[43] UNC1999 was developed as an analogue of GSK126, and was the first orally bioavailable EZH2 inhibitor to show activity. However, it is less selective than its counterpart GSK126, and it binds to EZH1 as well, increasing the potential for off-target effects.
Combination therapies are being studied as possible treatments when primary treatments begin to fail. Etoposide, a topoisomerase inhibitor, when combined with an EZH2 inhibitor, becomes more effective for non-small cell lung cancers with BRG1 and EGFR mutations.[35] However, EZH2 and lysine methylation can have tumor suppressing activity, for example in myelodysplastic syndrome,[47] indicating that EZH2 inhibition may not be beneficial in all cases.
Skeletal development
EZH2 is crucial for epigenetic regulation of specific patterning during osteochondrogenesis,[48] or bone and cartilage development, of the craniofacial skeletal elements. By repressing inhibitors, EZH2 promotes bone and cartilage formation in facial skeletal features arising from the neural crest. Above average EZH2 expression has become a biological marker for the most aggressive form for breast cancer known as Inflammatory Breast Cancer (IBC). But in 2013, a study performed by Zhaomei Mu and his associates concluded that the knockdown gene for EZH2 inhibited both the migration and invasion of IBC cells. Also in vivo, its knockdown gene suppressed tumor growth, most likely by the presence of fewer blood vessels, or reduced angiogenesis, in the EZH2 knockdown tumor versus EZH2 tumors.[49]
Weaver Syndrome
Mutations in the EZH2 gene have been linked with Weaver syndrome, a rare disorder characterized by advanced bone age, macrocephaly, and hypertelorism.[10] The histidine residue in the active site of the wild-type EZH2 was mutated to tyrosine in patients diagnosed with Weaver syndrome.[10] The mutation likely interferes with cofactor binding and causes disruption of the natural function of the protein.[10]
Taxonomic distribution

Enhancer of zeste (E(z)) was originally identified in Drosophila melanogaster, and its mammalian homologs were subsequently identified and named EZH1 (enhancer of zeste homolog 1) and EZH2 (enhancer of zeste homolog 2).[51] EZH2 is highly conserved through evolution. It and its homologs play essential roles in development, cell differentiation, and cell division in plants, insects, fish, and mammals.[13][17][52][53] The following taxonomic tree is a depiction of EZH2's distribution throughout a wide variety of species.[54][55]
See also
References
- ↑ 1.0 1.1 "The Polycomb group protein EZH2 directly controls DNA methylation". Nature 439 (7078): 871–874. February 2006. doi:10.1038/nature04431. PMID 16357870. Bibcode: 2006Natur.439..871V.
- ↑ "Role of histone H3 lysine 27 methylation in Polycomb-group silencing". Science 298 (5595): 1039–1043. November 2002. doi:10.1126/science.1076997. PMID 12351676. Bibcode: 2002Sci...298.1039C.
- ↑ "Polycomb group protein-mediated repression of transcription". Trends in Biochemical Sciences 35 (6): 323–332. June 2010. doi:10.1016/j.tibs.2010.02.009. PMID 20346678.
- ↑ "The Polycomb complex PRC2 and its mark in life". Nature 469 (7330): 343–349. January 2011. doi:10.1038/nature09784. PMID 21248841. Bibcode: 2011Natur.469..343M.
- ↑ "Targeting EZH2 in cancer". Nature Medicine 22 (2): 128–134. February 2016. doi:10.1038/nm.4036. PMID 26845405.
- ↑ "EZH2 methyltransferase and H3K27 methylation in breast cancer". International Journal of Biological Sciences 8 (1): 59–65. 2012. doi:10.7150/ijbs.8.59. PMID 22211105.
- ↑ "The polycomb group protein EZH2 is involved in progression of prostate cancer". Nature 419 (6907): 624–629. October 2002. doi:10.1038/nature01075. PMID 12374981. Bibcode: 2002Natur.419..624V.
- "Scientists Identify Gene That Marks Deadliest Form of Prostate Cancer". Scientific American. October 10, 2002. http://www.scientificamerican.com/article/scientists-identify-gene-2002-10-10/. Retrieved 2015-02-23.
- ↑ "The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors". Nature Communications 6. January 2015. doi:10.1038/ncomms7051. PMID 25609585. Bibcode: 2015NatCo...6.6051Z.
- ↑ "Increased expression of EZH2, a polycomb group protein, in bladder carcinoma". Urologia Internationalis 75 (3): 252–257. 2005. doi:10.1159/000087804. PMID 16215315.
- ↑ 10.0 10.1 10.2 10.3 "Mutations in EZH2 cause Weaver syndrome". American Journal of Human Genetics 90 (1): 110–118. January 2012. doi:10.1016/j.ajhg.2011.11.018. PMID 22177091.
- ↑ 11.0 11.1 "EZH2-mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia". Nature Neuroscience 16 (12): 1745–1753. December 2013. doi:10.1038/nn.3564. PMID 24162653.
- ↑ 12.0 12.1 12.2 12.3 12.4 Universal protein resource accession number Q15910 at UniProt.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 "EZH2: biology, disease, and structure-based drug discovery". Acta Pharmacologica Sinica 35 (2): 161–174. February 2014. doi:10.1038/aps.2013.161. PMID 24362326.
- ↑ 14.0 14.1 14.2 14.3 14.4 "EZH2 in normal and malignant hematopoiesis". Leukemia 28 (1): 44–49. January 2014. doi:10.1038/leu.2013.288. PMID 24097338.
- ↑ "RefSeq". https://cgwb.nci.nih.gov/cgi-bin/hgc?hgsid=518648&c=chr7&o=148504463&t=148581441&g=refGene&i=NM_001203247.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 "The polycomb protein Ezh2 impacts on induced pluripotent stem cell generation". Stem Cells and Development 23 (9): 931–940. May 2014. doi:10.1089/scd.2013.0267. PMID 24325319.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 "Inner workings and regulatory inputs that control Polycomb repressive complex 2". Chromosoma 121 (3): 221–234. June 2012. doi:10.1007/s00412-012-0361-1. PMID 22349693.
- ↑ 18.0 18.1 "Histone H3K27". EpiGenie. http://epigenie.com/key-epigenetic-players/histone-proteins-and-modifications/histone-h3k27/.
- ↑ "Heterochromatin revisited". Nature Reviews. Genetics 8 (1): 35–46. January 2007. doi:10.1038/nrg2008. PMID 17173056. https://zenodo.org/record/1233527. Retrieved 2018-11-04.
- ↑ 20.0 20.1 "Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment". Molecular and Cellular Biology 25 (7): 2525–2538. April 2005. doi:10.1128/MCB.25.7.2525-2538.2005. PMID 15767660.
- ↑ Epigenetics and Chromatin. Springer. 2008. ISBN 9783540852360. https://books.google.com/books?id=GbTGDgApUM8C&q=x-inactivation+ezh2&pg=PA108. Retrieved 2020-10-12.
- ↑ "Cell-extrinsic hematopoietic impact of Ezh2 inactivation in fetal liver endothelial cells". Blood 131 (20): 2223–2234. May 2018. doi:10.1182/blood-2017-10-811455. PMID 29555646.
- ↑ "Ezh2 is essential for the generation of functional yolk sac derived erythro-myeloid progenitors". Nature Communications 12 (1). December 2021. doi:10.1038/s41467-021-27140-8. PMID 34857757. Bibcode: 2021NatCo..12.7019N.
- ↑ 24.0 24.1 "Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA". Genes & Development 24 (23): 2615–2620. December 2010. doi:10.1101/gad.1983810. PMID 21123648.
- ↑ "CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing". Development 136 (18): 3131–3141. September 2009. doi:10.1242/dev.037127. PMID 19700617.
- ↑ "Age-correlated protein and transcript expression in breast cancer and normal breast tissues is dominated by host endocrine effects". Nature Cancer 1 (5): 518–532. May 2020. doi:10.1038/s43018-020-0060-4. PMID 35121983.
- ↑ "SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex". Molecular Cell 15 (1): 57–67. July 2004. doi:10.1016/j.molcel.2004.06.020. PMID 15225548.
- ↑ "Point mutations in the WD40 domain of Eed block its interaction with Ezh2". Molecular and Cellular Biology 18 (10): 5634–5642. October 1998. doi:10.1128/MCB.18.10.5634. PMID 9742080.
- ↑ 29.0 29.1 29.2 29.3 "Functional characterization of EZH2β reveals the increased complexity of EZH2 isoforms involved in the regulation of mammalian gene expression". Epigenetics & Chromatin 6 (1). February 2013. doi:10.1186/1756-8935-6-3. PMID 23448518.
- ↑ "The diverse functions of histone lysine methylation". Nature Reviews. Molecular Cell Biology 6 (11): 838–849. November 2005. doi:10.1038/nrm1761. PMID 16261189.
- ↑ "Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation". Nature Structural & Molecular Biology 19 (12): 1273–1281. December 2012. doi:10.1038/nsmb.2449. PMID 23160351.
- ↑ 32.0 32.1 "The SET-domain protein superfamily: protein lysine methyltransferases". Genome Biology 6 (8): 227. 2005. doi:10.1186/gb-2005-6-8-227. PMID 16086857.
- ↑ 33.0 33.1 33.2 "Enzyme-dependent lysine deprotonation in EZH2 catalysis". Biochemistry 52 (39): 6866–6878. October 2013. doi:10.1021/bi400805w. PMID 24000826.
- ↑ "Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation". Blood 117 (8): 2451–2459. February 2011. doi:10.1182/blood-2010-11-321208. PMID 21190999.
- ↑ 35.0 35.1 "Window of Vulnerability". Harvard Medical School. 28 January 2015. http://hms.harvard.edu/news/window-vulnerability.
- ↑ 36.0 36.1 "An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1". ACS Chemical Biology 8 (6): 1324–1334. 2013. doi:10.1021/cb400133j. PMID 23614352.
- ↑ "EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer". The EMBO Journal 22 (20): 5323–5335. October 2003. doi:10.1093/emboj/cdg542. PMID 14532106.
- ↑ "Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells". Molecular and Cellular Biology 27 (14): 5105–5119. July 2007. doi:10.1128/MCB.00162-07. PMID 17502350.
- ↑ "Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin". Nature Genetics 42 (2): 181–185. February 2010. doi:10.1038/ng.518. PMID 20081860.
- ↑ 40.0 40.1 "Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells". Genes & Development 21 (9): 1050–1063. May 2007. doi:10.1101/gad.1524107. PMID 17437993.
- ↑ 41.0 41.1 "A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells". Nature Chemical Biology 8 (11): 890–896. November 2012. doi:10.1038/nchembio.1084. PMID 23023262.
- ↑ 42.0 42.1 42.2 "Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation". Proceedings of the National Academy of Sciences of the United States of America 109 (52): 21360–21365. December 2012. doi:10.1073/pnas.1210371110. PMID 23236167. Bibcode: 2012PNAS..10921360Q.
- ↑ 43.0 43.1 "EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations". Nature 492 (7427): 108–112. December 2012. doi:10.1038/nature11606. PMID 23051747. Bibcode: 2012Natur.492..108M.
- ↑ 44.0 44.1 "Catalytic roles for carbon-oxygen hydrogen bonding in SET domain lysine methyltransferases". The Journal of Biological Chemistry 281 (28): 19280–19287. July 2006. doi:10.1074/jbc.M602257200. PMID 16682405.
- ↑ Epizyme Announced Clinical Data from Phase 1 Trial of EZH2 Inhibitor EPZ-6438 (E7438) to be Presented at EORTC-NCI-AACR Symposium. (2014, October 1).
- ↑ "FDA granted accelerated approval to tazemetostat for follicular lymphoma". FDA. 18 June 2020. https://www.fda.gov/drugs/fda-granted-accelerated-approval-tazemetostat-follicular-lymphoma. Retrieved 27 July 2020.
- ↑ "Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes". Nature Genetics 42 (8): 665–667. August 2010. doi:10.1038/ng.620. PMID 20601954.
- ↑ "Epigenetic Control of Skeletal Development by the Histone Methyltransferase Ezh2". The Journal of Biological Chemistry 290 (46): 27604–27617. November 2015. doi:10.1074/jbc.M115.672345. PMID 26424790.
- ↑ "EZH2 knockdown suppresses the growth and invasion of human inflammatory breast cancer cells". Journal of Experimental & Clinical Cancer Research 32 (1). September 2013. doi:10.1186/1756-9966-32-70. PMID 24294976.
- ↑ "Ensembl". http://www.ensembl.org/Homo_sapiens/Gene/Compara_Tree?db=core;g=ENSG00000106462;r=7:148807383-148884321;redirect=no.
- ↑ "Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres". The EMBO Journal 16 (11): 3219–3232. June 1997. doi:10.1093/emboj/16.11.3219. PMID 9214638.
- ↑ "NCBI UniGene". https://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=444082&ALLPROT=1.
- ↑ "GeneCards". https://www.genecards.org/cgi-bin/carddisp.pl?gene=EZH2&ortholog=all#orthologs.
- ↑ "Ensembl". http://dec2014.archive.ensembl.org/Homo_sapiens/Gene/Compara_Tree?db=core;g=ENSG00000106462;r=7:148807383-148884321;redirect=no.
- ↑ "Ensembl 2014". Nucleic Acids Research 42 (Database issue): D749–D755. January 2014. doi:10.1093/nar/gkt1196. PMID 24316576.
Further reading
- "The Polycomb group protein Enhancer of Zeste 2: its links to DNA repair and breast cancer". Journal of Molecular Histology 37 (5–7): 219–223. September 2006. doi:10.1007/s10735-006-9042-9. PMID 16855786.
- "Epigenetic control of hematopoietic stem cell aging the case of Ezh2". Annals of the New York Academy of Sciences 1106 (1): 233–239. June 2007. doi:10.1196/annals.1392.008. PMID 17332078. Bibcode: 2007NYASA1106..233D.
- "Interaction of Vav with ENX-1, a putative transcriptional regulator of homeobox gene expression". Molecular and Cellular Biology 16 (6): 3066–3073. June 1996. doi:10.1128/MCB.16.6.3066. PMID 8649418.
- "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research 6 (9): 791–806. September 1996. doi:10.1101/gr.6.9.791. PMID 8889548.
- "Characterization of EZH1, a human homolog of Drosophila Enhancer of zeste near BRCA1". Genomics 37 (2): 161–171. October 1996. doi:10.1006/geno.1996.0537. PMID 8921387.
- "Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres". The EMBO Journal 16 (11): 3219–3232. June 1997. doi:10.1093/emboj/16.11.3219. PMID 9214638.
- "Specific interaction between the XNP/ATR-X gene product and the SET domain of the human EZH2 protein". Human Molecular Genetics 7 (4): 679–684. April 1998. doi:10.1093/hmg/7.4.679. PMID 9499421.
- "Interaction of mouse polycomb-group (Pc-G) proteins Enx1 and Enx2 with Eed: indication for separate Pc-G complexes". Molecular and Cellular Biology 18 (6): 3572–3579. June 1998. doi:10.1128/MCB.18.6.3572. PMID 9584197.
- "Characterization of interactions between the mammalian polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian polycomb-group protein complexes". Molecular and Cellular Biology 18 (6): 3586–3595. June 1998. doi:10.1128/mcb.18.6.3586. PMID 9584199.
- "Point mutations in the WD40 domain of Eed block its interaction with Ezh2". Molecular and Cellular Biology 18 (10): 5634–5642. October 1998. doi:10.1128/MCB.18.10.5634. PMID 9742080.
- "Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation". Nature Genetics 23 (4): 474–478. December 1999. doi:10.1038/70602. PMID 10581039.
- "The human EZH2 gene: genomic organisation and revised mapping in 7q35 within the critical region for malignant myeloid disorders". European Journal of Human Genetics 8 (3): 174–180. March 2000. doi:10.1038/sj.ejhg.5200439. PMID 10780782.
- "Distinct BMI-1 and EZH2 expression patterns in thymocytes and mature T cells suggest a role for Polycomb genes in human T cell differentiation". Journal of Immunology 166 (10): 5925–5934. May 2001. doi:10.4049/jimmunol.166.10.5925. PMID 11342607.
- "Polycomblike PHD fingers mediate conserved interaction with enhancer of zeste protein". The Journal of Biological Chemistry 276 (46): 43065–43073. November 2001. doi:10.1074/jbc.M104294200. PMID 11571280.
- "The polycomb group protein EZH2 is involved in progression of prostate cancer". Nature 419 (6907): 624–629. October 2002. doi:10.1038/nature01075. PMID 12374981. Bibcode: 2002Natur.419..624V.
- "EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells". Proceedings of the National Academy of Sciences of the United States of America 100 (20): 11606–11611. September 2003. doi:10.1073/pnas.1933744100. PMID 14500907. Bibcode: 2003PNAS..10011606K.
 |
