Chemistry:Antimony potassium tartrate
| |
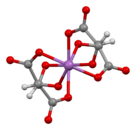
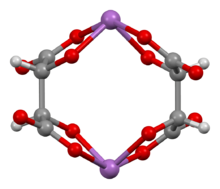 Ball-and-stick model of the bis(μ2-tartrato)-di-antimony anion,[1][2] [Sb2(C4H2O6)2]2−
Carbon Hydrogen Oxygen Antimony | |
| Names | |
|---|---|
| Other names
potassium antimonyl tartrate
emetic tartar tartar emetic | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 1332600 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| K2Sb2(C4H2O6)2 · 3 H2O | |
| Molar mass | 667.87 g/mol |
| Appearance | white crystalline powder |
| Density | 2.6 g/cm3 |
| 8.3 g/100 mL (0 °C) 35.9 g/100 mL (100 °C) | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
110 mg/kg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Antimony potassium tartrate, also known as potassium antimonyl tartrate, potassium antimontarterate, or tartar emetic,[3] has the formula K2Sb2(C4H2O6)2. The compound has long been known as a powerful emetic, and was used in the treatment of schistosomiasis and leishmaniasis. It is used as a resolving agent. It typically is obtained as a hydrate.
Medical
The first treatment application against trypanosomiasis was tested in 1906, and the compound's use to treat other tropical diseases was researched.[4] The treatment of leishmania with antimony potassium tartrate started in 1913. After the introduction of antimony(V) containing complexes like sodium stibogluconate and meglumine antimoniate, the use of antimony potassium tartrate was phased out.[4][5] After British physician John Brian Christopherson's discovery in 1918 that antimony potassium tartrate could cure schistosomiasis, the antimonial drugs became widely used.[6][7][8] However, the injection of antimony potassium tartrate had severe side effects such as Adams–Stokes syndrome[9] and therefore alternative substances were under investigation. With the introduction and subsequent larger use of praziquantel in the 1970s, antimony-based treatments fell out of use.[10][11]
Tartar emetic was used in the late 19th and early 20th century in patent medicine as a remedy for alcohol intoxication, and was first ruled ineffective in the United States in 1941, in United States v. 11 1/4 Dozen Packages of Articles Labeled in Part Mrs. Moffat's Shoo-Fly Powders for Drunkenness.[12][13]
The New England Journal of Medicine reported[14] a case study of a patient whose wife secretly gave him a dose of a product called "tartaro emetico" which contained trivalent antimony (antimony potassium tartrate) and is sold in Central America as an aversive treatment for alcohol use disorder. The patient, who had been out drinking the night before, developed persistent vomiting shortly after being given orange juice with the drug. When admitted to the hospital, and later in the intensive care unit, he experienced severe chest pains, cardiac abnormalities, renal and hepatic toxicity, and nearly died. The Journal reports that "Two years later, he [the patient] reports complete abstinence from alcohol."
Emetic

Antimony potassium tartrate's potential as an emetic has been known since the Middle Ages. The compound itself was considered toxic and therefore a different way to administer it was found. Cups made from pure antimony were used to store wine for 24 hours and then the resulting solution of antimony potassium tartrate in wine was consumed in small portions until the wanted emetic effect was reached.[15][16][17]
Poisoning by "tartarised antimony" or "emetic tartar" is a plot device in the first modern detective novel, The Notting Hill Mystery (1862). The emetic tartar was kept by a character in the novel because he was "addicted to the pleasures of the table, and was in the habit of taking an occasional emetic."[18]
The compound is still used to induce vomiting in captured animals in order to study their diets.[19][20][21]
Insecticide
Antimony potassium tartrate is used as an insecticide against thrips.[22] It is in IRAC class 8E.[23]
Preparation, structure, reactions
Antimony potassium tartrate is prepared by treating a solution of potassium hydrogen tartrate and antimony trioxide:
- 2 KOH + Sb2O3 + (HOCHCO2H)2 → K2Sb2(C4H2O6)2 + 3 H2O
With an excess of tartaric acid, the monoanionic monoantimony salt is produced:[2]
- 2 KOH + Sb2O3 + 4 (HOCHCO2H)2 → 2 KSb(C4H2O6)2 + 2 H2O
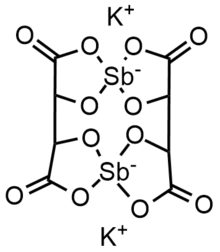
Antimony potassium tartrate has been the subject of several X-ray crystallography studies.[24][25][26][27][28][2] The core complex is an anionic dimer of antimony tartrate (Sb2(C4H2O6)22-) which is arranged in a large ring with the carbonyl groups pointing outwards. The complex has D2 molecular symmetry with two Sb(III) centers bonded in distorted square pyramids. Water and potassium ions are held within the unit cell but are not tightly bound to the dimer. The anion is a well-used resolving agent.[29]
Further reading
Of historic interest:
- Frederick, George Mann (1952). Practical organic chemistry.. England: Longmans, Green & Co.. p. 115. ISBN 0-582-44407-1. http://catalogue.bl.uk/primo_library/libweb/action/display.do?frbrVersion=6&tabs=moreTab&ct=display&fn=search&doc=BLL01014269928&indx=1&recIds=BLL01014269928&recIdxs=0&elementId=0&renderMode=poppedOut&displayMode=full&frbrVersion=6&dscnt=1&scp.scps=scope%3A%28BLCONTENT%29&frbg=&tab=local_tab&dstmp=1419325338798&srt=rank&mode=Basic&vl%28488279563UI0%29=any&dum=true&tb=t&vl%28freeText0%29=PRACTICAL%20ORGANIC%20CHEMISTRY&vid=BLVU1.
- Knapp, Fr. (1839). "Zur Bildungsgeschichte des Brechweinsteins". Annalen der Pharmacie 32: 76–85. doi:10.1002/jlac.18390320107. https://zenodo.org/record/1426943.
References
- ↑ Palenik, R.C.; Abboud, K.A.; Palenik, G.J. (2005). CSD Entry: DKSBTR02: Di-potassium bis(μ2-tartrato)-di-antimony trihydrate. Cambridge Crystallographic Data Centre. doi:10.5517/cc89ghh.
- ↑ 2.0 2.1 2.2 Palenik, Ruth C.; Abboud, Khalil A.; Palenik, Gus J. (March 2005). "Bond valence sums and structural studies of antimony complexes containing Sb bonded only to O ligands". Inorganica Chimica Acta 358 (4): 1034–1040. doi:10.1016/j.ica.2004.11.013.
- ↑ Sun, H.; Yan, S.C.; Cheng, W.S. (2003). "Interaction of antimony tartrate with the tripeptide glutathione". European Journal of Biochemistry 267 (17): 5450–5457. doi:10.1046/j.1432-1327.2000.01605.x. PMID 10951203.
- ↑ 4.0 4.1 Low, George C. (1916). "The history of the use of intravenous injections of tartar emetic (Antimonium tartaratum) in tropical medicine". Transactions of the Royal Society of Tropical Medicine and Hygiene 10 (2): 37–42. doi:10.1016/S0035-9203(16)90068-3.
- ↑ Navarro, Maribel; Gabbiani, Chiara; Messori, Luigi; Gambino, Dinorah (2010). "Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: Recent achievements and perspectives". Drug Discovery Today 15 (23–24): 1070–8. doi:10.1016/j.drudis.2010.10.005. PMID 20974285.
- ↑ Christopherson, J.B. (1918). "The Successful Use of Antimony in Bilharziosis". The Lancet 192 (4958): 325–327. doi:10.1016/S0140-6736(01)02807-0. https://zenodo.org/record/1907214.
- ↑ Crichton-Harris, Ann (2009-07-15). Poison in small measure: Dr. Christopherson and the cure for bilharzia. BRILL. ISBN 978-90-04-17541-9. https://books.google.com/books?id=L3OsNIofmHQC.
- ↑ Sabah, A.A.; Fletcher, Cathy; Webbe, G.; Doenhoff, M.J. (1986). "Schistosoma mansoni: Chemotherapy of infections of different ages". Experimental Parasitology 61 (3): 294–303. doi:10.1016/0014-4894(86)90184-0. PMID 3086114.
- ↑ t'Ao, S. C. (1957). "Cardiac manifestations of the toxic action of potassium antimony tartrate in schistosomiasis patients: Paroxysmal ventricular tachycardia and fibrillation". Chinese Medical Journal 75 (5): 365–378. PMID 13447130.
- ↑ Hagan, Paul (2009). "Schistosomiasis – a rich vein of research". Parasitology 136 (12): 1611–9. doi:10.1017/S003118200999093X. PMID 19691867.
- ↑ Publishers, Bentham Science (October 1996). "The Antimonials". Current Medicinal Chemistry. pp. 304–305. https://books.google.com/books?id=GlICpnVArb8C&pg=PA304.
- ↑ Silverman, Matt (2003). Loony lawsuits. Barnes & Noble Books. p. 81. ISBN 978-0-7607-3893-1. https://books.google.com/books?id=4xz4uVZbjtkC. Retrieved 1 January 2013.
- ↑ "United States v. 111/4 DOZEN PACKAGES, ETC....". https://scholar.google.com/scholar_case?case=3856056384057161206&hl=en&as_sdt=2&as_vis=1&oi=scholarr.
- ↑ Macías Konstantopoulos, Wendy; Burns Ewald, Michele; Pratt, Daniel S. (2012). "Case 22-2012: A 34-Year-Old Man with Intractable Vomiting after Ingestion of an Unknown Substance". New England Journal of Medicine 367 (3): 259–68. doi:10.1056/NEJMcpc1111580. PMID 22808962.
- ↑ McCallum, RI (1977). "President's address. Observations upon antimony". Proceedings of the Royal Society of Medicine 70 (11): 756–63. doi:10.1177/003591577707001103. PMID 341167.
- ↑ Thomson, SC (1926). "Antimonyall Cupps: Pocula Emetica or Calices Vomitorii". Proceedings of the Royal Society of Medicine 19 (Sect Hist Med): 122.2–128. doi:10.1177/003591572601901707. PMID 19985185.
- ↑ Weiss, S.; Hatcher, RA (1922). "The Mechanism of the Vomiting Induced by Antimony and Potassium Tartrate (Tartar Emetic)". Journal of Experimental Medicine 37 (1): 97–111. doi:10.1084/jem.37.1.97. PMID 19868716.
- ↑ Felix, Charles. "The Notting Hill Mystery" (in en). https://www.gutenberg.org/cache/epub/46153/pg46153-images.html.
- ↑ Poulin, B.; Lefebvre, G. t.; McNeil, R. (1994). "Effect and Efficiency of Tartar Emetic in Determining the Diet of Tropical Land Birds". The Condor 96 (1): 98–104. doi:10.2307/1369067.
- ↑ Carlisle, J. D.; Holberton, R. L. (2006). "Relative Efficiency of Fecal versus Regurgitated Samples for Assessing Diet and the Deleterious Effects of a Tartar Emetic on Migratory Birds". Journal of Field Ornithology 77 (2): 126–135. doi:10.1111/j.1557-9263.2006.00032.x.
- ↑ Diamond, Antony W.; Fayad, V. C.; McKinley, Peter S. (2007). "Commentary: Ipecac: An Improved Emetic for Wild Birds". Journal of Field Ornithology 78 (4): 436–439. doi:10.1111/j.1557-9263.2007.00136.x.
- ↑ Grout, N.J.S.; Stephen, T.G. (1 January 1996). "Citrus thrips (Thysanoptera: Thripidae) in Swaziland develop tolerance to tartar emetic bait". African Entomologist 4 (1): 15–20. https://hdl.handle.net/10520/AJA10213589_178.
- ↑ Sparks, Thomas C; Storer, Nicholas; Porter, Alan; Slater, Russell; Nauen, Ralf (2021). "Insecticide resistance management and industry: the origins and evolution of the I nsecticide R esistance A ction C ommittee (IRAC) and the mode of action classification scheme". Pest Management Science 77 (6): 2609–2619. doi:10.1002/ps.6254. ISSN 1526-498X. PMID 33421293. Bibcode: 2021PMSci..77.2609S.
- ↑ Grdenić, D.; Kamenar, B. (1 August 1965). "Structures involving unshared electron pairs: coordination of antimony in racemic potassium antimonyl tartrate". Acta Crystallographica 19 (2): 197–199. doi:10.1107/S0365110X65003080. Bibcode: 1965AcCry..19..197G.
- ↑ Banerjee, A.K.; Chari, K.V.R. (September 1969). "Structure of potassium antimony tartrate". Journal of Inorganic and Nuclear Chemistry 31 (9): 2958–2962. doi:10.1016/0022-1902(69)80218-6.
- ↑ Iyer, R.Krishna; Deshpande, S.G.; Rao, G.S. (November 1972). "Studies on complexes of tartaric acid—I". Journal of Inorganic and Nuclear Chemistry 34 (11): 3351–3356. doi:10.1016/0022-1902(72)80229-X.
- ↑ Kamenar, B.; Grdnić, D.; Prout, C. K. (31 March 1970). "The crystal and molecular structure of racemic potassium di-μ-tartrato-diantimonate(III) trihydrate (racemic 'tartar emetic')". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 26 (3): 181–188. doi:10.1107/S0567740870002133. PMID 5467310. Bibcode: 1970AcCrB..26..181K.
- ↑ Gress, Mary E.; Jacobson, Robert A. (January 1974). "X-ray and white radiation neutron diffraction studies of optically active potassium antimony tartrate, K2Sb2(d-C4H2O6)2·3H2O (tarter emetic)". Inorganica Chimica Acta 8: 209–217. doi:10.1016/S0020-1693(00)92617-3.
- ↑ F. P. Dwyer; F. L. Garvan (1960). "Resolution of cis -Dinitrobis(ethylenediamine)cobalt Ion". Resolution of cis-Dinitrobis(ethylenediamine)cobalt Ion. Inorganic Syntheses. 6. pp. 195–197. doi:10.1002/9780470132371.ch62. ISBN 978-0-470-13237-1.
Further reading
- Priesner, Claus (1997). "Basilius Valentinus und die Labortechnik um 1600". Berichte zur Wissenschaftsgeschichte 20 (2–3): 159–172. doi:10.1002/bewi.19970200205.
- Geoffroy, M.; Stack, T. (1751). "Observations on the Effects of the Vitrum Antimonii Ceratum, by Mons. Geoffroy, of the Royal Academy of Sciences, and F. R. S. Translated from the French by Tho. Stack, M. D. F. R. S". Philosophical Transactions 47: 273–278. doi:10.1098/rstl.1751.0042.
- Berzelius, Jöns Jacob (1824). Lehrbuch der Chemie. https://books.google.com/books?id=R4M5AAAAcAAJ&pg=PA196.
- Copus, Martinus (1569). Das Spißglas in ein Glas gegossen, das man Vitrum Antimonii nennt, ein wahrhafftige Gift vnd gantzgeferliche Artzney sey. https://books.google.com/books?id=i148AAAAcAAJ.
- The Technologist. 1861. https://books.google.com/books?id=XxMAAAAAMAAJ&pg=PA393.
- "Captain Cook's Antimony Cup". Vesalius, VII, 2: 62–64. 2001. http://www.bium.univ-paris5.fr/ishm/vesalius/VESx2001x07x02.pdf.
- Schneider, R. (1859). "Ueber einige Antimon-Verbindungen". Annalen der Physik und Chemie 184 (11): 407–415. doi:10.1002/andp.18591841104. Bibcode: 1859AnP...184..407S. https://zenodo.org/record/1423664.
- Groschuff, E. (1918). "Reines Antimon". Zeitschrift für anorganische und allgemeine Chemie 103: 164–188. doi:10.1002/zaac.19181030109. Bibcode: 1918ZAACh.103..164G. https://zenodo.org/record/1428160.
- Soubeiran, E.; Capitaine, H. (1840). "Zur Geschichte der Weinsteinsäure". Journal für Praktische Chemie 19: 435–442. doi:10.1002/prac.18400190171. https://zenodo.org/record/1427784.
- Pfaff, C. H. (1838). "Ueber Antimon-Wasserstoffgas und die davon abhängige Unsicherheit des von James Marsh entdeckten Verfahrens zur Entdeckung des Arseniks". Archiv der Pharmazie 64 (2): 169–174. doi:10.1002/ardp.18380640215. https://zenodo.org/record/1424517.
- Zimmermann, E. (1930). "Das Antimon in der Chemotherapie". Klinische Wochenschrift 9: 27–31. doi:10.1007/BF01740712.
- Gress, Mary E.; Jacobson, Robert A. (1974). "X-ray and white radiation neutron diffraction studies of optically active potassium antimony tartrate, K2Sb2(d-C4H2O6)2·3H2O (tarter emetic)". Inorganica Chimica Acta 8: 209–217. doi:10.1016/S0020-1693(00)92617-3.
 |