Chemistry:Dabrafenib
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tafinlar |
| Other names | GSK-2118436 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613038 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
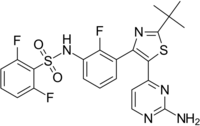
| Formula | C23H20F3N5O2S2 |
| Molar mass | 519.56 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dabrafenib, sold under the brand name Tafinlar among others, is an anti-cancer medication used for the treatment of cancers associated with a mutated version of the gene BRAF.[1] Dabrafenib acts as an inhibitor of the associated enzyme B-Raf, which plays a role in the regulation of cell growth.
The most common side effects include papilloma (warts), headache, nausea, vomiting, hyperkeratosis (thickening and toughening of the skin), hair loss, rash, joint pain, fever and tiredness.[2] When taken in combination with trametinib, the most common side effects include fever, tiredness, nausea, chills, headache, diarrhea, vomiting, joint pain and rash.[2]
Dabrafenib was approved for medical use in the United States in May 2013,[6] and in the European Union in August 2013.[2]
Medical uses
Dabrafenib is indicated as a single agent for the treatment of people with unresectable or metastatic melanoma with BRAF V600E mutation.[1] Dabrafenib is indicated, in combination with trametinib, for BRAF V600E-positive unresectable or metastatic melanoma, melanoma, metastatic non-small cell lung cancer, metastatic anaplastic thyroid cancer, and unresectable or metastatic solid tumors.[1][2][7]
History
Clinical trial data demonstrated that resistance to dabrafenib and other BRAF inhibitors occurs within six to seven months.[8] To overcome this resistance, the BRAF inhibitor dabrafenib was combined with the MEK inhibitor trametinib.[8] In January 2014, the FDA approved this combination of dabrafenib and trametinib for BRAF V600E/K-mutant metastatic melanoma.[9][10] In May 2018, the FDA approved the combination dabrafenib/trametinib as an adjuvant treatment for BRAF V600E-mutated, stage III melanoma after surgical resection based on the results of the COMBI-AD phase 3 study,[11] making it the first oral chemotherapy regimen that prevents cancer relapse for node positive, BRAF-mutated melanoma.[12]
Society and culture
Legal status
The US Food and Drug Administration approved dabrafenib as a single agent treatment for people with BRAF V600E mutation-positive advanced melanoma in May 2013.[6][13] Dabrafenib was approved for use in the European Union in August 2013.[2]
In April 2017, the European Union approved the combination of dabrafenib with trametinib for BRAF V600-positive advanced or metastatic non small-cell lung cancer (NSCLC).[14][15][2]
In September 2023, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Finlee, intended for the treatment of low- and high-grade glioma (LGG and HGG).[16] The applicant for this medicinal product is Novartis Europharm Limited.[16] Finlee was approved for medical use in the European Union in November 2023.[3]
Brand names
Dabrafenib is the international nonproprietary name.[17]
Dabrafenib is sold under the brand names Tafinlar[2] and Finlee.[3]
Research
Dabrafenib has clinical activity with a manageable safety profile in clinical trials of phase I and II in patients with BRAF (V600)-mutated metastatic melanoma.[18][19]
References
- ↑ 1.0 1.1 1.2 1.3 "Tafinlar- dabrafenib capsule". DailyMed. U.S. National Library of Medicine. June 22, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fee1e6b1-e1a5-4254-9f2e-a70e0f8dbdea.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Tafinlar EPAR". September 17, 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/tafinlar. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 3.0 3.1 3.2 "Finlee EPAR". November 15, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/finlee.
- ↑ "Tafinlar Product information". 29 August 2013. https://ec.europa.eu/health/documents/community-register/html/h865.htm.
- ↑ "Finlee Product information". 16 November 2023. https://ec.europa.eu/health/documents/community-register/html/h1767.htm.
- ↑ 6.0 6.1 "Drug Approval Package: Tafinlar (dabrafenib) Capsules NDA #202806". December 24, 1999. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202806Orig1s000TOC.cfm.
- ↑ "FDA approves dabrafenib with trametinib for pediatric patients with low-grade glioma with a BRAF V600E mutation". March 16, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-trametinib-pediatric-patients-low-grade-glioma-braf-v600e-mutation.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 8.0 8.1 "Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations". The New England Journal of Medicine 367 (18): 1694–1703. November 2012. doi:10.1056/NEJMoa1210093. PMID 23020132.
- ↑ "Dabrafenib/Trametinib Combination Approved for Advanced Melanoma". OncLive. January 9, 2013. http://www.onclive.com/web-exclusives/FDA-Approves-First-Ever-Combination-for-Metastatic-Melanoma.
- ↑ "Metastatic melanoma - a review of current and future treatment options". Acta Dermato-Venereologica 95 (5): 516–524. May 2015. doi:10.2340/00015555-2035. PMID 25520039.
- ↑ "Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma". The New England Journal of Medicine 377 (19): 1813–1823. November 2017. doi:10.1056/NEJMoa1708539. PMID 28891408.
- ↑ "FDA Approves Adjuvant Combo for BRAF+ Melanoma". WebMD LLC. https://www.medscape.com/viewarticle/895984.
- ↑ "GSK melanoma drugs add to tally of U.S. drug approvals". Reuters. May 30, 2013. https://www.reuters.com/article/us-glaxosmithkline-approvals-idUSBRE94S1A020130530.
- ↑ "EU Approves Dabrafenib/Trametinib Combination in BRAF+ NSCLC". April 4, 2017. https://www.targetedonc.com/view/eu-approves-dabrafenibtrametinib-combination-in-braf-nsclc.
- ↑ "Mekinist EPAR". September 17, 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/mekinist.
- ↑ 16.0 16.1 "Finlee: Pending EC decision". September 15, 2023. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/finlee. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 67". WHO Drug Information 26 (1): 45–96. 2012.
- ↑ "Clinical development of dabrafenib in BRAF mutant melanoma and other malignancies". Expert Opinion on Drug Metabolism & Toxicology 9 (7): 893–899. July 2013. doi:10.1517/17425255.2013.794220. PMID 23621583.
- ↑ "B-Raf and the inhibitors: from bench to bedside". Journal of Hematology & Oncology 6: 30. April 2013. doi:10.1186/1756-8722-6-30. PMID 23617957.
Further reading
- "Dabrafenib Therapy and BRAF and G6PD Genotype". Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). 2017. Bookshelf ID: NBK447415. https://www.ncbi.nlm.nih.gov/books/NBK447415/. Retrieved February 5, 2020.
 |
