Chemistry:Ellagic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3,7,8-Tetrahydroxy[1]benzopyrano[5,4,3-cde][1]benzopyran-5,10-dione | |
| Other names
4,4′,5,5′,6,6′-Hexahydroxydiphenic acid 2,6,2′,6′-dilactone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H6O8 | |
| Molar mass | 302.197 g/mol |
| Density | 1.67 g/cm3 |
| Melting point | >360 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

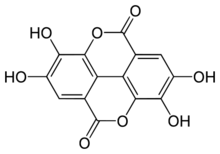
Ellagic acid is a polyphenol found in numerous fruits and vegetables. It is the dilactone of hexahydroxydiphenic acid.
Name
The name comes from the French term acide ellagique, from the word galle spelled backward[1] because it can be obtained from noix de galle (galls), and to distinguish it from acide gallique (gallic acid). The molecular structure resembles to that of two gallic acid molecules being assembled "head to tail" and bound together by a C–C bond (as in biphenyl, or in diphenic acid) and two lactone links (cyclic carboxylic esters).
Metabolism
Biosynthesis
Plants produce ellagic acid from hydrolysis of tannins such as ellagitannin and geraniin.[2]
Biodegradation
Urolithins are gut flora human metabolites of dietary ellagic acid derivatives.[3][4] Ellagic acid has low bioavailability, with 90% remaining unabsorbed from the intestines until metabolized by microflora to the more bioavailable urolithins.[4]
History
Ellagic acid was first discovered by chemist Henri Braconnot in 1831.[5] Maximilian Nierenstein prepared this substance from algarobilla, dividivi, oak bark, pomegranate, myrabolams, and valonea in 1905.[5] He also suggested its formation from galloyl-glycine by Penicillium in 1915.[6] Julius Löwe was the first person to synthesize ellagic acid by heating gallic acid with arsenic acid or silver oxide.[5][7]
Natural occurrences
Ellagic acid is found in edible nuts.[8] It is also found in oak species such as the North American white oak (Quercus alba) and European red oak (Quercus robur).[9]
The macrophyte Myriophyllum spicatum produces ellagic acid.[10]
Ellagic acid can be found in the medicinal mushroom Phellinus linteus.[11]
In food
The highest levels of ellagic acid are found in raw chestnuts, walnuts, pecans, cranberries, raspberries, strawberries, and grapes, as well as distilled beverages.[12] It is also found in peaches[13] and pomegranates.[14]
| Dietary source | Ellagic acid[15] |
|---|---|
| Fruits (mg/100g fresh weight) | |
| Yellow raspberries | 1900 |
| Cloudberries | 315.1 |
| Raspberries | 270 |
| Pomegranate | 269.9[16] |
| Blackberries | 150 |
| Rose hip | 109.6 |
| Black raspberries | 90 |
| Strawberries | 77.6 |
| Boysenberries | 70 |
| Strawberry jam | 24.5 |
| Nuts (mg/100g fresh weight) | |
| Walnuts | 59 |
| Pecans | 33 |
| Beverages (mg/L) | |
| Pomegranate juice | 811.1[16] |
| Cognac | 31–55 |
| Oak-aged red wine | 33 |
| Whiskey | 1.2 |
| Seeds (mg/g) | |
| Boysenberries | 30 |
| Red raspberries | 8.7 |
| Black raspberries | 6.7 |
| Mango | 1.2 |
Research and health claims
Ellagic acid has been marketed as a dietary supplement with various claimed benefits against cancer, heart disease, and other diseases. In the 21st century, numerous U.S.-based supplement companies received FDA warning letters for promoting ellagic acid with false anti-disease claims that violate the Federal Food, Drug, and Cosmetic Act.[17][18][19] Ellagic acid has been identified by the FDA as a "fake cancer 'cure'".[18] There is no scientific evidence to support the claims that ellagic acid can treat or prevent cancer.[18]
See also
- List of ineffective cancer treatments
References
- ↑ Littré, Émile. "ellagique". http://artflx.uchicago.edu/cgi-bin/dicos/pubdico1look.pl?strippedhw=ellagique&dicoid=LITTRE1872.
- ↑ Seigler, David S. (December 31, 1998). Plant Secondary Metabolism. Springer Science & Business Media. p. 208. ISBN 978-0-412-01981-4. https://books.google.com/books?id=hmYbjbGU-EgC.
- ↑ Larrosa, M.; González Sarrías, A.; García Conesa, M. T.; Tomás Barberán, F. A.; Espín, J. C. (2006). "Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities". Journal of Agricultural and Food Chemistry 54 (5): 1611–1620. doi:10.1021/jf0527403. PMID 16506809. Bibcode: 2006JAFC...54.1611L.
- ↑ 4.0 4.1 "Bioactivity of dietary polyphenols: The role of metabolites". Critical Reviews in Food Science and Nutrition 60 (4): 626–659. 2020. doi:10.1080/10408398.2018.1546669. PMID 30614249.
- ↑ 5.0 5.1 5.2 Grasser, Georg; Enna, F. G. A. (1922). Synthetic Tannins. Read Books. p. 20. ISBN 9781406773019. https://archive.org/details/synthetictannins032496mbp.
- ↑ Nierenstein, M. (1915). "The Formation of Ellagic Acid from Galloyl-Glycine by Penicillium". The Biochemical Journal 9 (2): 240–244. doi:10.1042/bj0090240. PMID 16742368.
- ↑ Löwe, Julius (1868). "Über die Bildung von Ellagsäure aus Gallussäure". Zeitschrift für Chemie 4: 603.
- ↑ Bodoira, Romina; Maestri, Damián (2020). "Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity". Journal of Agricultural and Food Chemistry 68 (4): 927–942. doi:10.1021/acs.jafc.9b07160. PMID 31910006. Bibcode: 2020JAFC...68..927B.
- ↑ Mämmelä, P.; Savolainen, H.; Lindroos, L.; Kangas, J.; Vartiainen, T. (2000). "Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry". Journal of Chromatography A 891 (1): 75–83. doi:10.1016/S0021-9673(00)00624-5. PMID 10999626.
- ↑ Nakai, S. (2000). "Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa". Water Research 34 (11): 3026–3032. doi:10.1016/S0043-1354(00)00039-7. Bibcode: 2000WatRe..34.3026N.
- ↑ Lee, Y.-S.; Kang, Y.-H.; Jung, J.-Y.; Lee, S.; Ohuchi, K.; Shin, K.-H.; Kang, I.-J.; Park, J.-H. et al. (2008). "Protein glycation inhibitors from the fruiting body of Phellinus linteus". Biological and Pharmaceutical Bulletin 31 (10): 1968–1972. doi:10.1248/bpb.31.1968. PMID 18827365.
- ↑ Vattem, D. A.; Shetty, K. (2005). "Biological Function of Ellagic Acid: A Review". Journal of Food Biochemistry 29 (3): 234–266. doi:10.1111/j.1745-4514.2005.00031.x.
- ↑ Infante, R.; Contador, L.; Rubio, P.; Aros, D.; Peña Neira, Á. (2011). "Postharvest sensory and phenolic characterization of 'Elegant Lady' and 'Carson' peaches". Chilean Journal of Agricultural Research 71 (3): 445–451. doi:10.4067/S0718-58392011000300016. http://www.scielo.cl/pdf/chiljar/v71n3/at16.pdf.
- ↑ Usta, C.; Özdemir, S.; Schiariti, M.; Puddu, P. E. (November 2013). "The pharmacological use of ellagic acid-rich pomegranate fruit". International Journal of Food Sciences and Nutrition 64 (7): 907–913. doi:10.3109/09637486.2013.798268. PMID 23700985.
- ↑ Landete, J.M. (2011). "Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health". Food Research International 44 (5): 1150–1160. doi:10.1016/j.foodres.2011.04.027.
- ↑ 16.0 16.1 García-Villalba, Rocío; Espín, Juan Carlos; Tomás-Barberán, Francisco A. (2016). "Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid". Journal of Chromatography A 1428: 162–175. doi:10.1016/j.chroma.2015.08.044. PMID 26341594.
- ↑ "Warning letter:VitaPurity Corporation". US Food and Drug Administration. May 12, 2017. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/vitapurity-corporation-514472-05122017.
- ↑ 18.0 18.1 18.2 "187 Fake Cancer 'Cures' Consumers Should Avoid". U.S. Food and Drug Administration. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm.
- ↑ "FDA Cracks Down On Unproved Cancer Cures". CBS News. June 17, 2008. https://www.cbsnews.com/news/fda-cracks-down-on-unproved-cancer-cures/.
Template:Unproven and disproven cancer treatments
 |
