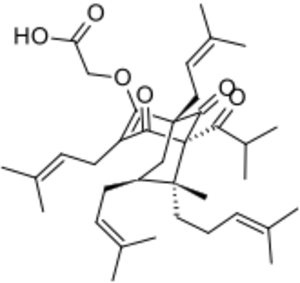
Chemistry:Hyperforin
 | |
 | |
| Clinical data | |
|---|---|
| Dependence liability | None |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic and CYP3A & CYP2B |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C35H52O4 |
| Molar mass | 536.797 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 79–80 °C (174–176 °F) |
| Solubility in water | 0.66 mg/mL (20 °C) |
| |
| |
| | |
Hyperforin is a phytochemical produced by some of the members of the plant genus Hypericum, notably Hypericum perforatum (St John's wort).[2] Hyperforin may be involved in the pharmacological effects of St. John's wort,[2] specifically in its antidepressant effects.[3][4][5]
Occurrence
Hyperforin has only been found in significant amounts in Hypericum perforatum with other related species such as Hypericum calycinum containing lower levels of the phytochemical.[2] It accumulates in oil glands, pistils, and fruits, probably as a plant defensive compound.[6] The first natural extractions were done with ethanol and afforded a 7:1 yield of crude extract to phytochemical however, this technique produced a mixture of hyperforin and adhyperforin.[3][7][8] The extraction technique has since been modernized using lipophilic liquid CO2 extraction to afford a 3:1 crude to phytochemical extraction which is then further purified away from adhyperforin.[3][7][8] This CO2 extraction is rather tricky still because typical 'supercritical' conditions extract less material whereas anything over 40 °C (100 °F) will degrade hyperforin.[3][7][8] Other Hypericum species contain low amounts of hyperforin.[9]
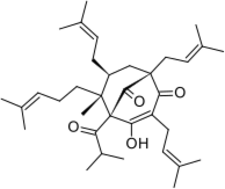
Chemistry
Hyperforin is a prenylated phloroglucinol derivative and is a member of the Polycyclic polyprenylated acylphloroglucinol family, also known as the PPAP family. Hyperforin is a unique PPAP because it consists of a C8 quaternary stereocenter which was a synthetic challenge unlike other PPAP synthetic targets.[3][4][10] The structure of hyperforin was elucidated by a research group from the Shemyakin Institute of Bio-organic Chemistry (USSR Academy of Sciences in Moscow) and published in 1975.[11][12] A total synthesis of the non-natural hyperforin enantiomer was reported in 2010 which required approximately 50 synthetic transformations.[13] In 2010, an enantioselective total synthesis of the correct enantiomer was disclosed. The retrosynthetic analysis was inspired by hyperforin's structural symmetry and biosynthetic pathway. The synthetic route undertaken generated a prostereogenic intermediate which then established the synthetically challenging C8 stereocenter and facilitated the stereochemical outcomes for the remainder of the synthesis.[10]
Hyperforin is unstable in the presence of light and oxygen.[14] Frequent oxidized forms contain a C3 to C9 hemiketal/heterocyclic bridge or will form furan/pyran derivatives.[7][8]
Pharmacokinetics
Some pharmacokinetic data on hyperforin is available for an extract containing 5% hyperforin. Maximal plasma levels (Cmax) in human volunteers were reached 3–4 hours after administration of an extract containing 14.8 mg hyperforin. Biological half-life (t1/2) and mean residence time were 9 hours and 12 hours, respectively, with an estimated steady state plasma concentration of 100 ng/mL (approx. 180 nM) for 3 doses per day. Linear plasma concentrations were observed within a normal dosage range and no accumulation occurred.[15]
In healthy male volunteers, 612 mg dry extract of St. John's wort produced hyperforin pharmacokinetics characterised by a half life of 19.64 hours.[16]
Pharmacodynamics
Hyperforin may be a constituent responsible for the antidepressant and anxiolytic properties of the extracts of St. John's wort.[2][17] In vitro, it acted as a reuptake inhibitor of monoamines (MRI), including serotonin, norepinephrine, dopamine, and of GABA and glutamate, with IC50 values of 0.05-0.10 μg/mL for all compounds, with the exception of glutamate, which is in the 0.5 μg/mL range.[18] In other laboratory studies, hyperforin induced cytochrome P450 enzymes CYP3A4 and CYP2C9 by binding to and activating the pregnane X receptor.[19]
| Neurotransmitter | IC50 (nanomoles)[18] |
|---|---|
| Norepinephrine | 80 ± 24 |
| Dopamine | 102 ± 19 |
| GABA | 184 ± 41 |
| 5-HT | 205 ± 45 |
| Glutamate | 829 ± 687 |
| Choline | 8500 |
| Receptor | Ki (nanomoles) |
|---|---|
| D1 | 595.8[20] |
Hyperforin Biosynthesis
Hyperforin is a polyprenylated acylphloroglucinol (PPAP) derivative with a pholoroisobutyrophenone bicyclic core. Isobutryl-CoA (17) has been determined to be one of the initial primary metabolite starter molecules in the biosynthesis of the hyperforin core structure. Isobutryl-CoA is derived from an a-ketoisovalerate intermediate (15). The bicyclic structure suggests that it has elements of meroterpenoid origin. The nucleus of hyperforin is formed in a sequence condensation of one molecule of isobutyryl-CoA and three molecules of malonyl-CoA, both catalyzed by Isobutyrophenone synthase. Type III PKS enzymes will catalyze the decarboxylative condensation of enzyme active sites to generate scaffolding.
These enzymes preferred a different substrate and did not produce identical products. The cell-free extracts from the cell cultures were incubated with isobutyryl-CoA and malonyl-CoA, phlorisobutyrophenone was formed (18). The enzymatic reaction was identified as BUS. PIVP is a similar function of enzyme in glandular hairs of hop cones. Two acylphloroglucinoal cores PICP and PIBP formed are formed by claisen condensation but will differ in substrate and enzyme specificities. PIVP will use isovaleryl-CoA in the presence of an enzyme VSP, and PIBP will use isobutyryl-CoA in the presence of bus resulting in the production of adhyperforin and hyperforin.
However, hyperforin is an easily degradable compound highly sensitive to heat and light in its powder form or within a solution, making it difficult to determine a true synthesis route for hyperforin making this synthesis route a possible route.[21]
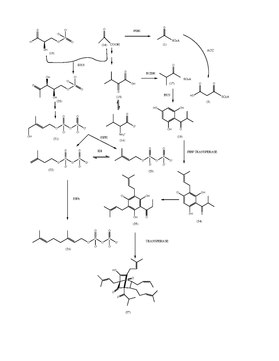
- Natural and semi-synthetic analogues of Hyperforin
-
Aristoforin
-
Hyperforin trimethoxybenzoate
-
Tetrahydrohyperforin
-
Hyperforin nicotinate
Antidepressant research
Two meta-analyses of preliminary clinical trials evaluating the efficacy of St. John's wort for treating mild-to-moderate depression indicated a response similar to selective serotonin reuptake inhibitors and with better tolerance, although the long-term generalization of study results was limited by the short duration (4–12 weeks) of reviewed studies.[22][23]
See also
References
- ↑ St John's Wort available again . Irishhealth.com (2015-10-13). Retrieved on 2020-02-11.
- ↑ 2.0 2.1 2.2 2.3 "Hyperforin". PubChem, US National Library of Medicine. 8 September 2018. https://pubchem.ncbi.nlm.nih.gov/compound/441298#section=Top.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Biotechnological production of hyperforin for pharmaceutical formulation". European Journal of Pharmaceutics and Biopharmaceutics 126: 10–26. May 2018. doi:10.1016/j.ejpb.2017.03.024. PMID 28377273.
- ↑ 4.0 4.1 "Polycyclic polyprenylated acylphloroglucinols". Chemical Reviews 106 (9): 3963–3986. September 2006. doi:10.1021/cr0500582. PMID 16967926.
- ↑ "Hyperforin depletes synaptic vesicles content and induces compartmental redistribution of nerve ending monoamines". Life Sciences 75 (23): 2841–2850. October 2004. doi:10.1016/j.lfs.2004.08.004. PMID 15464835.
- ↑ "Hyperforin". Phytochemistry 67 (20): 2201–2207. October 2006. doi:10.1016/j.phytochem.2006.08.017. PMID 16973193. Bibcode: 2006PChem..67.2201B.
- ↑ 7.0 7.1 7.2 7.3 "Further degradation product of hyperforin from Hypericum perforatum (St John's Wort)". Fitoterapia 74 (5): 439–444. July 2003. doi:10.1016/S0367-326X(03)00114-X. PMID 12837358.
- ↑ 8.0 8.1 8.2 8.3 "Furohyperforin, a prenylated phloroglucinol from st. John's wort (Hypericumperforatum)". Journal of Natural Products 62 (5): 770–772. May 1999. doi:10.1021/np980470v. PMID 10346967.
- ↑ "Phytochemical analysis of nine Hypericum L. species from Serbia and the F.Y.R. Macedonia". Die Pharmazie 61 (3): 251–252. March 2006. PMID 16599273.
- ↑ 10.0 10.1 "Enantioselective total synthesis of hyperforin". Journal of the American Chemical Society 135 (2): 644–647. January 2013. doi:10.1021/ja312150d. PMID 23270309. http://nrs.harvard.edu/urn-3:HUL.InstRepos:12563735.
- ↑ "[Structure of the antibiotic hyperforin]" (in ru). Doklady Akademii Nauk SSSR 226 (1): 88–90. January 1976. PMID 1248360.
- ↑ "[The structure of hyperforin]". Tetrahedron Letters 16 (32): 2791–2794. 1975. doi:10.1016/S0040-4039(00)75241-5.
- ↑ "Catalytic asymmetric total synthesis of ent-hyperforin". Angewandte Chemie 49 (6): 1103–1106. February 2010. doi:10.1002/anie.200906678. PMID 20063336.
- ↑ "Liquid chromatography-mass spectrometry studies of St. John's wort methanol extraction: active constituents and their transformation". Journal of Pharmaceutical and Biomedical Analysis 37 (2): 303–312. February 2005. doi:10.1016/j.jpba.2004.10.034. PMID 15708671.
- ↑ "Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers". Pharmacopsychiatry 31 (Suppl 1): 36–43. June 1998. doi:10.1055/s-2007-979344. PMID 9684946.
- ↑ "Investigation of the bioavailability of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin following single and multiple oral dosing of a hypericum extract containing tablet". Arzneimittel-Forschung 55 (1): 15–22. 2005. doi:10.1055/s-0031-1296820. PMID 15727160.
- ↑ Herbal medicines: a guide for healthcare professionals. London: Pharmaceutical Press. 2002. ISBN 978-0-85369-474-8.
- ↑ 18.0 18.1 "Hyperforin as a possible antidepressant component of hypericum extracts". Life Sciences 63 (6): 499–510. 1998. doi:10.1016/S0024-3205(98)00299-9. PMID 9718074.
- ↑ "St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor". Proceedings of the National Academy of Sciences of the United States of America 97 (13): 7500–7502. June 2000. doi:10.1073/pnas.130155097. PMID 10852961. Bibcode: 2000PNAS...97.7500M.
- ↑ "Hyperforin". http://www.bindingdb.org/bind/searchby_r1l.jsp?constrain=0&reactant1=hyperforin&tag=r1l&loMW=&hiMW=&loKI=&hiKI=&loIC=&hiIC=&lodG=&hidG=&anDor=and&submit=Search.
- ↑ "The Biochemical and Genetic Basis for the Biosynthesis of Bioactive Compounds in Hypericum Perforatum L., One of the Largest Medicinal Crops in Europe". Genes 11 (10): 1210. October 2020. doi:10.3390/genes11101210. PMID 33081197.
- ↑ "Clinical use of Hypericum perforatum (St John's wort) in depression: A meta-analysis". Journal of Affective Disorders 210: 211–221. March 2017. doi:10.1016/j.jad.2016.12.048. PMID 28064110. "27 clinical trials with a total of 3808 patients were reviewed [...] For patients with mild-to-moderate depression, St John's wort has comparable efficacy and safety when compared to SSRIs. Follow-up studies carried out over a longer duration should be planned to ascertain its benefits.".
- ↑ "A meta-analysis on the efficacy and safety of St John's wort extract in depression therapy in comparison with selective serotonin reuptake inhibitors in adults". Neuropsychiatric Disease and Treatment 12: 1715–1723. 2016. doi:10.2147/NDT.S106752. PMID 27468236. "A total of 3,126 patients with depression were included. St John’s wort extract did not differ from SSRIs in clinical response, remission, and mean reduction in Hamilton Rating Scale for Depression score. [...] Both St John’s wort extract and SSRIs are effective in treating mild-to-moderate depression. St John’s wort extract is safer than SSRIs.".
External links
 |