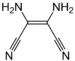Chemistry:List of chemical compounds with unusual names
Chemical nomenclature, replete as it is with compounds with complex names, is a repository for some names that may be considered unusual. A browse through the Physical Constants of Organic Compounds in the CRC Handbook of Chemistry and Physics (a fundamental resource) will reveal not just the whimsical work of chemists, but the sometimes peculiar compound names that occur as the consequence of simple juxtaposition. Some names derive legitimately from their chemical makeup, from the geographic region where they may be found, the plant or animal species from which they are isolated or the name of the discoverer.
Some are given intentionally unusual trivial names based on their structure, a notable property or at the whim of those who first isolate them. However, many trivial names predate formal naming conventions. Trivial names can also be ambiguous or carry different meanings in different industries, geographic regions and languages.
Godly noted that "Trivial names having the status of INN or ISO are carefully tailor-made for their field of use and are internationally accepted".[1] In his preface to Chemical Nomenclature, Thurlow wrote that "Chemical names do not have to be deadly serious".[2] A website in existence since 1997[3] and maintained at the University of Bristol lists a selection of "molecules with silly or unusual names" strictly for entertainment. These so-called silly or funny trivial names (depending on culture) can also serve an educational purpose. In an article in the Journal of Chemical Education, Dennis Ryan argues that students of organic nomenclature (considered a "dry and boring" subject) may actually take an interest in it when tasked with the job of converting funny-sounding chemical trivial names to their proper systematic names.[4]
The collection listed below presents a sample of trivial names and gives an idea how chemists are inspired when they coin a brand new name for a chemical compound outside of systematic naming. It also includes some examples of systematic names and acronyms that accidentally resemble English words.
Elements
Glenn Seaborg told his students that he proposed the chemical symbol Pu (from P U) instead of the conventional "Pl" for plutonium as a joke, only to find it officially adopted.[5] Unununium (Uuu) was the former temporary name of the chemical element number 111, a synthetic transuranium element. This element was named roentgenium (Rg) in November 2004.
Compounds
Name based on shape
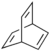
| Barrelene | C8H8, the name derives from the resemblance to a barrel.[6] |
| Basketane | pentacyclo[4.4.0.02,5.03,8.04,7]decane (C10H12), a polycyclic alkane with a structure similar to a basket.[3] |
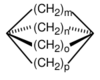
| Churchane | A polycyclic alkane named "churchane" because it looks superficially like a church. |
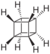
| Cubane | A hydrocarbon whose eight carbon atoms occupy the vertices of a cube.[7] |
| Dodecahedrane | A Platonic hydrocarbon shaped like a dodecahedron.[8] |
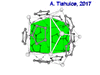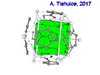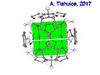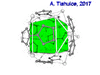
| Fenestrane (Windowpane) | A class of compounds with a "window pane motif" (the name fenestrane derives from the Latin word fenestra, meaning window), comprising four fused carbocycles centred on a quaternary carbon resulting in a twice-over spiro compound. The illustration at right shows a generic fenestrane as well as the specific example [4,4,4,4]fenestrane. Fenestranes are of considerable interest in theoretical chemistry though comparatively few have actually been synthesised. |
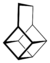
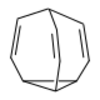
| Housane | A polycyclic alkane named "housane" because it looks superficially like a house.[3] |
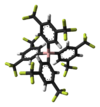
| Hitler's acid | Alternative name is Orthocarbonic acid (methanetetrol). The compound has also been given the nickname of "Hitler's Acid" due to the Ball-and-stick model of the compound resembling the Swastika symbol. |
| Ladderane | An organic molecule that looks like a ladder because it contains two or more fused rings of cyclobutane. |
| Nanokid | Nanokids belongs to a series of NanoPutians, a series of organic molecules whose structural formula resemble human forms. "NanoPutian" is a portmanteau of nano-, a unit prefix meaning one billionth, and lilliputian, a fictional race of humans in the novel Gulliver's Travels by Jonathan Swift.
There are no chemical or practical uses for the NanoKid molecule or any of its known derivatives. However, James Tour has turned the NanoKid into a lifelike character to educate children in the sciences. It was found that 82% of students found that NanoKids made learning science more interesting and led to a 10–59% increase in understanding of the material presented.[9] |
| Olympiadane | A mechanically-interlocked compound based on the topology for the Olympic rings. |
| Olympicene | Refers to the fused 5-benzene rings (C19H12), which is reminiscent of the Olympic Flag.[10] |
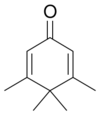
| Penguinone | 3,4,4,5-tetramethylcyclohexa-2,5-dienone; a two-dimensional representation of its structure resembles a penguin. |
| Prismane | An isomer of benzene with the carbon atoms arranged in the shape of a triangular prism. |
| Paddlanes | Paddlanes are bicyclic cyclohexane molecules that resemble the paddles on Steamboats. |
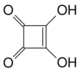
| Quadratic acid | A square-shaped organic compound, also called squaric acid. |
| Sulflower | A stable heterocyclic octacirculene based on thiophene, named as a portmanteau of sulfur and sunflower. |
| Volleyballene | Molecule composed of 60 carbon and 20 scandium atoms, which has an appearance similar to that of a volleyball. |
Named after people
| Buckminsterfullerene (Fullerene) | Also called the buckyball, this is an allotrope of carbon named after Richard Buckminster Fuller due to its resemblance to Fuller's geodesic domes. The term was coined by Harold Kroto.[11] The alternative name footballene was coined by A.D.J. Haymet[12] because the molecule also resembles a football;[3] the 70-atom version is said to resemble a rugby ball from its own oval shape. |
| Bullvalene | (tricyclo[3.3.2.02,8]deca-3,6,9-triene) (C10H10), was named by organic chemist Maitland Jones Jr. for William "Bull" Doering who predicted its properties in 1963.[13][14] Within a specific temperature range the molecule is subject to rapid degenerate Cope rearrangements with the result that all carbon atoms and hydrogen atoms are equivalent and that none of the carbon–carbon bonds is permanent. |
| Dickite | (Al 2Si 2O 5(OH) 4), a clay-like material with a number of manufacturing uses, one of which is as a coating for high-quality bond paper. It is named after its discoverer, Allan Brugh Dick.[15] |
| Josiphos ligands | A well-known catalyst, named after Josi Puleo, the technician who first prepared it.[16] Mandyphos and Taniaphos also exist. |
| Welshite |
A mineral named after the US amateur mineralogist Wilfred R. Welsh. Its formula is Ca2SbMg4FeBe2Si4O20. |
Named after fictional characters
| Alcindoromycine | An anthracycline antibiotic agent named after the character Alcindoro in La Bohème.[17] |
| Bohemamine | An anti-tumour agent named after the Puccini opera La Bohème.[17] |
| Collinemycin | An anthracycline antibiotic agent named after the character Colline in La Bohème.[17] |
| Ranasmurfin | A blue protein from the foam nests of a tropical frog, named after the Smurfs. |
| Mimimycin | An anthracycline antibiotic agent named after the character Mimì in La Bohème.[17] |
| Musettamycin | An anthracycline antibiotic agent named after the character Musetta in La Bohème.[17] |
| Marcellomycin | An anthracycline antibiotic agent named after the character Marcello in La Bohème.[17] |
| Pikachurin | A retinal protein named after Pokémon character / species Pikachu |
| Rudolphomycin | An anthracycline antibiotic agent named after the character Rodolfo (Rudolph) in La Bohème.[17][18] |
| Sonic hedgehog | A protein named after Sonic the Hedgehog |
Related to sex
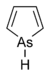
| Arsole | (C4H5As), an analogue of pyrrole in which an arsenic atom replaces the nitrogen atom.[19] The aromaticity of arsoles has been debated for many years.[20] The compound in which a benzene ring is fused to arsole — typically on the carbon atoms 3 and 4 — is known as benzarsole.[3] |
| Cumene | (C9H12), an aromatic hydrocarbon used in the production of phenol and acetone. |
| Cummingtonite | ((Mg,Fe2+)2(Mg,Fe2+)5Si8O22(OH)2), a magnesium-iron silicate hydroxide, first identified in Cummington, Massachusetts.[3] |
| FAP | Tris(pentafluoroethyl)trifluorophosphate, an anion used in some ionic liquids.[21] |
| Fornacite | A rare lead, copper chromate arsenate hydroxide mineral (Pb2CuCrO4AsO4OH), named after its discoverer, Lucien Lewis Forneau.[3] |
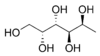
| Fucitol | (C6H14O5), an alcohol derived from Fucus vesiculosus, a North Atlantic seaweed. Its optical isomers are also called D-fuc-ol and L-fuc-ol.[3] |
| FucK | The name of the gene that encodes L-fuculokinase, an enzyme that catalyzes a chemical reaction between L-fuculose, ADP, and L-fuculose-1-phosphate.[3] |
| Fukalite | (Ca 4Si 2O 6(CO 3)(OH, F)) 2, a rare form of calcium silicocarbonate discovered in the Fuka Mine of Takahashi, Japan.[3] |
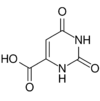
| Orotic acid | Pyrimidinecarboxylic acid has been referred to as vitamin B13. Often misspelled "erotic acid".[3] |
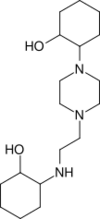
| Pizda | Abbreviated ligand name of a substance 1-(2’’-hydroxyl cyclohexyl)-3-[aminopropyl]-4-[3’-aminopropyl] piperazine, first synthesized by a group of Australia n chemists. In Romanian and some Slavic countries, the word pizda is a vulgarism for "vagina" (see Reconstruction:Proto-Slavic/pizda). |
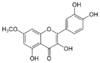
| Rhamnetin | A flavonol dye derived from buckthorn (rhamnus).[22] |
| SEX | An abbreviation of sodium ethyl xanthate,[23] a flotation agent used in the mining industry. |
| Spermine, Spermidine |
growth factors involved in cellular metabolism.[3] |
Related to bodily functions
| BARF | (tetrakis[3,5-bis(trifluoromethyl)phenyl]borate), a fluoroaryl borate B(Ar(CF3)2)4−, used as a non-coordinating anion[24] |
| catP | The name of the enzyme responsible for chloramphenicol resistance in various species of bacteria. |
| Constipatic acid | [2-(14-hydroxypentadecyl)-4-methyl-5-oxo-2,5-dihydrofuran-3-carboxylic acid], an aliphatic acid derived from the Australian Xanthoparmelia lichen.[3][25] |
| Crapinon | An anticholinergic drug, one side effect of which is constipation.[3] |
| Diurea | Organic compounds containing two urea units. Specific examples include methylene diurea and ethylene diurea. |
| dUMP | Deoxyuridine monophosphate, an intermediate in nucleotide metabolism |
| Earthcide, or Fartox |
Some of the many names for pentachloronitrobenzene, a fungicide.[26] |
| Nonanal | (C9H18O), an aldehyde derived from nonane.[27] |
| PoO | Chemical formula of polonium monoxide. |
| Uranate | The chemical term for an oxyanion of the element uranium.[3] |
| Vomitoxin | A mycotoxin occurring in grains.[3] |
Related to death and decay
| Cadaverine | A foul-smelling diamine produced by putrefaction of dead animal tissue.[3][28] |
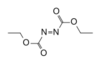
| DEAD, DEADCAT | Diethyl azodicarboxylate: An apt acronym, given that diethyl azodicarboxylate is explosive; shock sensitive; carcinogenic; and an eye, skin, and respiratory irritant.[3] |
| Earthcide, or Fartox |
Some of the many names for pentachloronitrobenzene, a fungicide.[29] |
| Putrescine | A foul-smelling diamine produced by the putrefaction of dead animal tissue.[3] |
Related to religion or legend
| Angelic acid | An organic acid found in garden angelica (Angelica archangelica), Umbelliferae, and many other plants. |
| DAMN | Diaminomaleonitrile, a cyanocarbon that contains two amine groups and two nitrile groups bound to an ethylene backbone. |
| Diabolic acid | A series of long-chain dicarboxylic acids with chains of different lengths. Named after the Greek word diabollo meaning to mislead.[30] |
| Draculin | An anticoagulant found in the saliva of vampire bats.[31] |
| Vitamin C (Godnose) | Albert Szent-Gyorgyi coined the term "ignose" to describe ascorbic acid, which he isolated and published. When the journal's editor refused to accept ignose as a plausible name, Szent-Gyorgyi suggested 'Godnose' (a joke meaning that only God could know the real identity of the molecule). The editor suggested that the name be changed to something more formal. [32] |
| Luciferase | A generic term for the class of oxidative enzymes that produce bioluminescence. |
| Lucifer yellow | Lucifer Yellow is a food coloring that is commonly found in hot sauces such as salsa pickle. Because it fluoresces under ultraviolet light and stains certain regions between plant cells, it's also used in plant microscopy anatomy studies. |
| Miraculin | A glycoprotein found in miracle fruit that makes sour foods taste sweet after contact with taste buds.[33] |
Sounds like a name (person, brand or organization)
| Adamantane | (tricyclo[3.3.1.13,7]decane), a crystalline cycloalkane,[34][35] an isomer of twistane. Name resembles that of English pop star Adam Ant.[3] |
| Irene | Hantzsch-Widman nomenclature for a monocyclic, heterocyclic compound with three ring atoms.[36] |
| Naftazone | (C11H9N3O2), a vasoprotective drug. The NAFTA free-trade zone is the area covered by the North American Free Trade Agreement.[37] |
| PEPPSI | Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation.[38] |
A part sounds like an English word
| Antipain | Antipain works as a protease inhibitor, preventing proteins from being degraded. It is a highly toxic compound that, ironically, causes severe itching or pain when it comes into contact with the skin. Because it inhibits the action of papain, an enzyme found in papayas, its name is actually an abbreviation of anti-papain. |
| Bongkrek acid | Name sounds like a combination of English words related to recreational drugs: bong; crack, a preparation of cocaine; and acid, a street name for lysergic acid diethylamide. |
| Constipatic Acid | Some Australian lichens, like Parmelia constipata, have this as a component. Protoconstipatic acid, dehydroconstipatic acid, and methyl constipatate are all constipatic acid derivatives. |
| Gardenin | Gardenins, which are flavones extracted from the Indian plant Gardenia lucida, come in a variety of forms. |
| Hirsutene | [39][40] Is also named after an animal, a goat (Hircus). |
| Magic acid | A superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). |
| Megaphone | A ketone derived from the root of Aniba megaphylla.[41] |
| Mispickel | An older name for the mineral arsenopyrite, an iron arsenic sulfide and major source of the element arsenic, sounds like 'miss pickle'. From German.[42] |
| Moronic acid | [3-oxoolean-18-en-28-oic acid], a natural triterpene |
| Noggin | A signalling protein involved in embryonic development. |
| Performic acid | A strongly oxidizing acid related to formic acid. |
| Periodic acid | Or per-iodic acid, is pronounced /ˌpɜːraɪˈɒdɪk/ PURR-eye-OD-ik and not */ˌpɪəriˈɒdɪk/ PEER-ee-OD-ik. It refers to one of two interconvertible species: HIO4 (metaperiodic acid), or H5IO6 (orthoperiodic acid – illustrated at right). The per- prefix in the name denotes that iodine is present in its highest possible (+VII) oxidation state. |
| Picket Fence Porphyrin | (5,10,15,20-tetrakis(alpha,alpha,alpha-2-pivalamidophenyl)porphyrin), used to model heme enzyme active sites. |
| Piranha solution | A strongly oxidizing mixture of hydrogen peroxide and sulfuric acid used to remove organic residues from substrates and glassware. The name refers to the voracious appetite of the Amazonian piranha fish. |
| Rednose | A sugar derived from the degradation of rudolphomycin.[17] |
| Rhamnose | A sugar naturally occurring in buckthorn (rhamnus). |
| Ru(Tris)BiPy-on-a-stick | Shorthand form of (trans-1,4-bis[(4-pyridyl)ethenyl]benzene)(2,2'-bipyridine)ruthenium(II).[43] |
| Traumatic acid | A substance occurring in plants, with a role in healing damaged tissue. |
Other
| Bastardane | A close relative to tetramantane (a higher homologue of adamantane), its proper name is nonacyclo[11.7.1.112,18.03,16.04,13.05,10.06,14.07,11.015,20]docosane. Because its unusual ethano-bridge was a deviation from the standard hydrocarbon caged rearrangements, it came to be known as bastardane—the unwanted child.[3][44] |
| Dinocap | (C18H24N2O6), a miticide and contact fungicide used to control powdery mildew in crops. |
| DuPhos | A class of asymmetric ligands for asymmetric synthesis. The name DuPhos is derived from the chemical company that developed this type of ligand (DuP, DuPont) and the compound class of phospholanes (Phos) it belongs to. |
| Homocubane | cubane homolog with additional CH2 group, C9H10 |
| Furfuryl Furfurate | The name of the molecule is difficult to say fast. It has a strong odor and can be used as a polymerization inhibitor in the vapor phase. Its name is derived from the Latin word "furfur," which means "bran" (the source of the compound). Furfural alcohol, a related molecule, is reportedly used in the fabrication of the Reinforced Carbon-Carbon (RCC) sections used in the space shuttle. |
| Methionylthreonylthreonylglutaminylarginyl...isoleucine | The IUPAC name for Titin. This is the largest known protein and so has the longest chemical name. Written in full, it contains 189,819 letters.[45] |
| Periplanone B | A pheromone of the female American cockroach. |
| Thebacon | Dihydrocodeinone enol acetate, an opioid analgesic or antitussive. [citation needed] |
| FOOF | Dioxygen difluoride, O2F2, an extremely unstable compound which reacts explosively with most other substances – the nickname "FOOF" is a play on its formula.[46] |
| Gossypol | A toxin found in cottonseed used as a male oral contraceptive.[3] |
| Melon | A compound consisting mostly of linked heptazine units with an undetermined composition. |
| DiNOsar | Common shortening of di-nitro sarcophagine. Used due to shorter length compared to the IUPAC name of 1,8-dinitro-3-6-10-13-16-19-hexaazabicyclo-[6.6.6]icosane. Sounds similar to the word Dinosaur |
See also
- International Union of Pure and Applied Chemistry
- IUPAC nomenclature
- List of places with unusual names
- List of unusual biological names
- List of places used in the names of chemical elements
References
- ↑ Godly, E.W. (1998). Chemical Nomenclature. Kluwer Academic Publishers. p. 20. ISBN 978-0-7514-0475-3.
- ↑ Thurlow, Kevin (1998). Chemical Nomenclature. Kluwer Academic Publishers. pp. xii. ISBN 978-0-7514-0475-3.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 May, Paul (28 May 2013). "Molecules with Silly or Unusual Names". Bristol University. http://www.chm.bris.ac.uk/sillymolecules/sillymols.htm.
- ↑ Ryan, Dennis (1997). "Old MacDonald Named a Compound: Branched Enynenynols". Journal of Chemical Education 74 (7): 782. doi:10.1021/ed074p782. Bibcode: 1997JChEd..74..782R. http://www.jce.divched.org/Journal/Issues/1997/Jul/PlusSub/V74N07/p782.pdf. Retrieved 2007-08-16.[yes|permanent dead link|dead link}}]
- ↑ Glenn T. Seaborg, Citizen-Scholar , By Peggy House, Reprinted from The Seaborg Center Bulletin, April 1999
- ↑ Zimmerman, Howard E.; Robert M. Paufler (1960). "Bicyclo [2.2.2]octa-2,5,7-triene (barrelene), a unique cyclic six electron pi system". Journal of the American Chemical Society 82 (6): 1514–1515. doi:10.1021/ja01491a071.
- ↑ Verbrugge, P. A. (1977). "Unusual organic compounds. XXIV. Compounds with the formula (CH)n. (d). Synthesis of cubane, (CH)8; homocubanes". Chemie en Techniek (Amsterdam) 32 (4): 120–123.
- ↑ Pubchem. "Dodecahedrane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/dodecahedrane#section=Related-Records.
- ↑ "NanoKids - Mission". 2007. Archived from the original on 2016-08-19. https://web.archive.org/web/20160819103912/http://cohesion.rice.edu/naturalsciences/nanokids/mission.cfm?doc_id=3039. Retrieved February 21, 2023.
- ↑ "'Olympic rings' molecule olympicene in striking image". BBC Online. 27 May 2012. https://www.bbc.co.uk/news/science-environment-18206015.
- ↑ Kroto, H.W.; Heath, J.R.; O'Brien, S.C.; Curl, R.F.; Smalley, R.E. (1985). "C60: Buckminsterfullerene". Nature 318 (6042): 162. doi:10.1038/318162a0. Bibcode: 1985Natur.318..162K.
- ↑ Haymet, A.D.J. (1986). "Footballene: a theoretical prediction for the stable, truncated icosahedral molecule C60". J. Am. Chem. Soc. 108 (2): 319. doi:10.1021/ja00262a035.
- ↑ Doering, W. von E.; Roth, W. R. (1963). "A Rapidly Reversible Degenerate Cope Rearrangement : Bicyclo[5.1.0]octa-2,5-diene". Tetrahedron 19 (5): 715–737. doi:10.1016/S0040-4020(01)99207-5.[|permanent dead link|dead link}}]
- ↑ Ault, Addison (2001). "The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure". J. Chem. Educ. 78 (7): 924. doi:10.1021/ed078p924. Bibcode: 2001JChEd..78..924A.
- ↑ Ross, C.; Kerr, P.F. (1931). "Dickite, a Kaolin Mineral". American Mineralogist 15: 34–39. http://www.minsocam.org/ammin/AM15/AM15_34.pdf.
- ↑ Blaser, Hans-Ulrich; Brieden, Walter; Pugin, Benoit; Spindler, Felix; Studer, Martin; Togni, Antonio (2002). "Solvias Josiphos ligands: from discovery to technical applications". Topics in Catalysis 19 (1): 3–16. doi:10.1023/A:1013832630565.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 Nettleton DE Jr, Balitz DM, Doyle TW, Bradner WT, Johnson DL, O'Herron FA, Schreiber RH, Coon AB, Moseley JE, Myllymaki RW, J Nat Prod. 1980 Mar–Apr;43(2):242–258. DOI: 10.1021/np50008a003
- ↑ "Canadian Patents Database CA 1110562: Anthracycline antibiotic designated RUDOLPHOMYCIN". http://patents1.ic.gc.ca/details?patent_number=1110562.
- ↑ G. Märkl; H. Hauptmann (1983-06-14). "Untersuchungen zur Chemie der Arsole 1,1-dichlor-1-R-λ5-arsole-1-chlorarsole 2,2′,5,5′-tetraphenyldiarsolyl (Studies on the chemistry of arsoles)". J. Organomet. Chem. 248 (3): 269–285. doi:10.1016/S0022-328X(00)98709-6.
- ↑ Mikael P. Johansson; Jonas Jusélius (2005). "Arsole Aromaticity Revisited". Lett. Org. Chem. (3): 469–474. http://www.helsinki.fi/~mpjohans/science/abstracts/008-abstract.html.
- ↑ Ignat’ev, N.V.; Welz-Biermann, U.; Kucheryna, A.; Bissky, G.; Willner, H. (2005). "New ionic liquids with tris(perfluoroalkyl)trifluorophosphate (FAP) anions" (in en). Journal of Fluorine Chemistry 126 (8): 1150–1159. doi:10.1016/j.jfluchem.2005.04.017. https://linkinghub.elsevier.com/retrieve/pii/S0022113905001570.
- ↑ Uri J, Csoban G, Viragh E., Acta Physiol Hung. 1951;2(2):223-8.
- ↑ See, for example, Okibe, N; Johnson, DB (2004). "Toxicity of flotation reagents to moderately thermophilic bioleaching microorganisms". Biotechnology Letters 24 (23): 2011–2016. doi:10.1023/A:1021118915720.
- ↑ "BARF". ChemSpider. Royal Society of Chemistry. 2013. http://www.chemspider.com/Chemical-Structure.2058460.html.
- ↑ Chester, DO (1979). "Three New Aliphatic Acids from Lichens of Genus Parmelia (Subgenus Xanthoparmelia )". Australian Journal of Chemistry 32 (11): 2565. doi:10.1071/CH9792565.
- ↑ "NIST Standard Reference Database 69, June 2005 Release: NIST Chemistry WebBook – Pentachloronitrobenzene". http://webbook.nist.gov/cgi/cbook.cgi?Name=fart*&Units=SI&cTG=on&cTC=on&cTP=on&cTR=on.
- ↑ May also be considered to be related to sex.
- ↑ Nordenström, Björn E. W. (1951). "Effect of cadaverine and lysine on the urinary excretion of piperidine in rabbits". Acta Pharmacologica et Toxicologica 7 (3): 287–296. doi:10.1111/j.1600-0773.1951.tb02870.x. PMID 14856760.
- ↑ "NIST Standard Reference Database 69, June 2005 Release: NIST Chemistry WebBook – Pentachloronitrobenzene". http://webbook.nist.gov/cgi/cbook.cgi?Name=fart*&Units=SI&cTG=on&cTC=on&cTP=on&cTR=on.
- ↑ R A Klein, G P Hazlewood, P Kemp, and R M Dawson, Biochem J. 1979 December 1; 183(3): 691–700.
- ↑ "Purification and partial characterization of draculin, the anticoagulant factor present in the saliva of vampire bats (Desmodus rotundus)". Thromb. Haemost. 73 (1): 94–100. 1995. doi:10.1055/s-0038-1653731. PMID 7740503. https://cris.maastrichtuniversity.nl/en/publications/de5bbfd2-4f82-454a-a6a7-0befd8bb65af.
- ↑ De Tullio, Mario C. (2012). "Beyond the antioxidant: the double life of vitamin C". Sub-Cellular Biochemistry 56: 49–65. doi:10.1007/978-94-007-2199-9_4. ISBN 978-94-007-2198-2. ISSN 0306-0225. PMID 22116694. https://pubmed.ncbi.nlm.nih.gov/22116694/.
- ↑ "Complete purification and characterization of the taste-modifying protein, miraculin, from miracle fruit". J. Biol. Chem. 263 (23): 11536–11539. August 1988. doi:10.1016/S0021-9258(18)37991-2. PMID 3403544.
- ↑ Prelog, V., Seiwerth, R. (1941). "Über eine neue, ergiebigere Darstellung des Adamantans". Berichte 74 (11): 1769–1772. doi:10.1002/cber.19410741109.
- ↑ Not to be confused with the fictional material adamantium
- ↑ Parent Hydride Names and Substantive Nomenclature. IUPAC. March 2004. p. 16. http://old.iupac.org/reports/provisional/abstract04/RB-prs310804/Chap6-3.04.pdf.
- ↑ "[Prevention of hemorrhage in prostatic surgery. Apropos of the study of the hemostatic activity in prostatectomy of a new molecule: beta-naphthoquinone monosemicarbazone (Naftazone)]" (in fr). Annales d'Urologie 6 (3): 209–212. 1972. PMID 4562066.
- ↑ "PEPPSI Catalysts". http://www.sigmaaldrich.com/chemistry/chemical-synthesis/technology-spotlights/peppsi.html.
- ↑ Curran, Dennis P. (1985). "Tandem radical approach to linear condensed cyclopentanoids. Total synthesis of (.+-.)-hirsutene". Journal of the American Chemical Society 107 (5): 1448–1449. doi:10.1021/ja00291a077.
- ↑ Nozoe, Shigeo (1976). "Isolation, structure and synthesis of hirsutene, a precursor hydrocarbon of coriolin biosynthesis". Tetrahedron Letters 17 (3): 195–198. doi:10.1016/0040-4039(76)80013-5.
- ↑ SM Kupchan, KL Stevens, EA Rohlfing, BR Sickles, AT Sneden, RW Miller, RF Bryan, J. Org. Chem., 43(4) (1978) 586
- ↑ Kouřimský, Jiří (1974). A Colour Guide to Familiar Minerals and Rocks. Octopus Books. p. 94. ISBN 0706404084.
- ↑ Toma, SH; Uemi, M; Nikolaou, S; Tomazela, DM; Eberlin, MN; Toma, HE (2004). "{trans-1,4-Bis[(4-pyridyl)ethenyl]benzene}(2,2'-bipyridine)ruthenium(II) Complexes and Their Supramolecular Assemblies with β-Cyclodextrin". Inorg Chem. 43 (11): 3521–3527. doi:10.1021/ic0352250. PMID 15154817.
- ↑ Schleyer, Paul von Rague; Eiji Osawa; Michael G. B. Drew (1968). "Nonacyclo[11.7.1.12,18.03,16.04,13.05,10.06,14.07,11.015,20docosane, a bastard tetramantane"]. J. Am. Chem. Soc. 90 (18): 5034–5036. doi:10.1021/ja01020a053. http://www.chm.bris.ac.uk/sillymolecules/bastardane.pdf. Retrieved 2007-08-15.
- ↑ Sam Kean (2011), The Disappearing Spoon, Little, Brown, p. 36, ISBN 9781446437650
- ↑ Derek, Lowe (2010-02-23). "Things I Won't Work With: Dioxygen Difluoride" (in en-US). https://www.science.org/content/blog-post/things-i-won-t-work-dioxygen-difluoride.
Bibliography
- E.C. Alyea, "Metal Complexes of Ditertiary Arsines. Chapter in Transition Metal Complexes of Phosphorus, Arsenic and Antimony Ligands", MacMillan, 1973. Chapter: "Some amusing names of arsine ligands: edas, vdias, dam, ffars etc"
- J. Andraos, "Glossary of Coined Names & Terms Used in Science", York University, 2004.
- Giles, P.M. (1999). "Revised Section F: Natural products and related compounds". Pure Appl. Chem. 71 (4): 587–643. doi:10.1351/pac199971040587. http://www.chem.qmul.ac.uk/iupac/sectionF/. Retrieved 2007-08-16.
- Aronson, Jeff (1999). "That's show business". British Medical Journal 319 (7215): 972. doi:10.1136/bmj.319.7215.972. PMID 10514162.
- Browne, Malcolm W. (April 22, 1986). "Chemists dabble in whimsy". International New York Times. https://www.nytimes.com/1986/04/22/science/chemists-dabble-in-whimsy.html.
- Paul May, "Molecules with Silly or Unusual Names," Imperial College Press, July 2008, ISBN:978-1-84816-207-5.
- Metanomski, W. V. (1987). "Unusual Names Assigned to chemical substances". Chem. Int. 9: 211–215.
- Monk, Felonious (13 April 2006). "Chemical Cock-ups: A Story of How Not to Name a Chemical Compound". h2g2: The guide to life, the universe and everything. Not Panicking Ltd. http://h2g2.com/approved_entry/A10176987.
- Alex Nickon and Ernest F. Silversmith, "Organic Chemistry, the Name Game: Modern Coined Terms and Their Origins", Pergamon 1987. ISBN:0-08-034481-X.
- Wallechinsky, David; Wallace, Amy (2005). "24. Molecules and amoebas with funny names". The new book of lists : the original compendium of curious information. New York, N.Y.: Canongate. pp. 203–205. ISBN 9781841957197.