Chemistry:PD-102,807
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
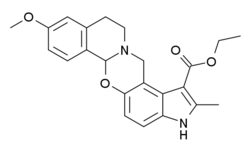
| Formula | C23H24N2O4 |
| Molar mass | 392.455 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
PD-102,807 is a drug which acts as a selective antagonist for the muscarinic acetylcholine receptor M4.[1][2][3] It is used in scientific research for studying the effects of the different muscarinic receptor subtypes in the body and brain.[4][5][6][7]
See also
References
- ↑ "PD 102807, a novel muscarinic M4 receptor antagonist, discriminates between striatal and cortical muscarinic receptors coupled to cyclic AMP". Life Sciences 65 (21): 2233–40. 1999. doi:10.1016/S0024-3205(99)00488-9. PMID 10576595.
- ↑ "Diphenidol-related diamines as novel muscarinic M4 receptor antagonists". Bioorganic & Medicinal Chemistry Letters 18 (9): 2972–6. May 2008. doi:10.1016/j.bmcl.2008.03.061. PMID 18395442.
- ↑ "Synthesis and pharmacology of benzoxazines as highly selective antagonists at M(4) muscarinic receptors". Journal of Medicinal Chemistry 45 (14): 3094–102. July 2002. doi:10.1021/jm011116o. PMID 12086495.
- ↑ "Pharmacological characterization of muscarinic receptor subtypes mediating vasoconstriction of human umbilical vein". British Journal of Pharmacology 147 (5): 516–23. March 2006. doi:10.1038/sj.bjp.0706654. PMID 16444291.
- ↑ "Muscarinic type 1 receptors mediate part of nitric oxide's vagal facilitatory effect in the isolated innervated rat right atrium". Nitric Oxide: Biology and Chemistry 16 (1): 110–7. February 2007. doi:10.1016/j.niox.2006.05.005. PMID 16843016.
- ↑ "The detection of the non-M2 muscarinic receptor subtype in the rat heart atria and ventricles". Naunyn-Schmiedeberg's Archives of Pharmacology 378 (1): 103–16. July 2008. doi:10.1007/s00210-008-0285-8. PMID 18443764.
- ↑ "Muscarinic modulation of synaptic transmission via endocannabinoid signalling in the rat midbrain periaqueductal gray". Molecular Pharmacology 74 (5): 1392–8. November 2008. doi:10.1124/mol.108.045872. PMID 18678620.
 |

