Chemistry:Adafenoxate
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
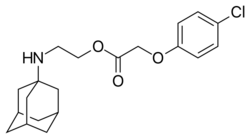
2-[(Adamantan-1-yl)amino]ethyl (4-chlorophenoxy)acetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H26ClNO3 | |
| Molar mass | 363.87834 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Adafenoxate is a compound related to centrophenoxine, that has been found to act as a nootropic in rats.[1]
Synthesis
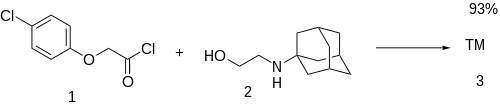
Ex 1: 4-Chlorophenoxyacetic acid (pCPA) [122-88-3] is converted to its acid chloride to give 4-chlorophenoxyacetyl chloride [4122-68-3] (1). Esterification with 2-(1-adamantylamino)ethanol [3716-66-3] (2) gives Adafenoxate (3) in a single step.
Ex 2: Same as above but Fischer–Speier esterification done via a DS-trap. This gives an 88% yield.
References
- ↑ "A study of nootropic drugs for anti-anxiety action". Acta Physiol Pharmacol Bulg 13 (4): 25–30. 1987. PMID 2896427.
- ↑ Romeo R. Andreoli, Xavier D. Cirera, U.S. Patent 4,476,319 (1984 to Sociedad Espanola De Especialidades Farmaco-Terapeuticas S.A.).
 |
