Chemistry:Trithionic acid
From HandWiki
| |
| Names | |
|---|---|
| IUPAC names
Thiodisulfuric acid, 1,5-dihydrido-2,2,4,4-tetraoxido-1,5-dioxy-2,3,4-trisulfy-[5]catena, trithionic acid[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| H 2O 6S 3[1][2][3] | |
| Molar mass | 194.19 g·mol−1[3] |
| Density | 2.4±0.1 g/cm3 |
| Melting point | 324.41 °C (615.94 °F; 597.56 K) |
| Boiling point | 739.35 °C (1,362.83 °F; 1,012.50 K) |
| 1e+006 mg/L | |
| log P | -1.1[2] |
| Vapor pressure | 1.07E-016 Pa (8.05E-019 mm Hg) |
| Acidity (pKa) | -6.94[2] |
| Conjugate base | Hydrogen trithionate |
Refractive index (nD)
|
1.700 |
| Pharmacology | |
| Pharmacokinetics: | |
| 0.570 hours | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
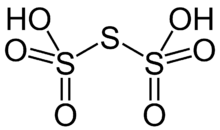
Trithionic acid is a polythionic acid with three sulfur atoms. It can be viewed as two bisulfite radicals bridged by a sulfur atom.
References
- ↑ 1.0 1.1 EBI Web Team. "trithionic acid (CHEBI:29210)" (in en). https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:29210. Retrieved 23 September 2018.
- ↑ 2.0 2.1 2.2 "Compound Report Card". https://www.ebi.ac.uk/chembl/compound/inspect/CHEMBL3754893. Retrieved 23 September 2018.
- ↑ 3.0 3.1 "Trithionic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/Trithionic_acid. Retrieved 23 September 2018.
 |
