Chemistry:Setoperone
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
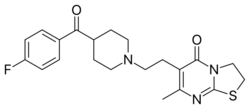
6-{2-[4-(4-Fluorobenzoyl)piperidin-1-yl]ethyl}-7-methyl-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyrimidin-5-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H24FN3O2S | |
| Molar mass | 401.50 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Setoperone is a compound that is a ligand to the 5-HT2A receptor. It can be radiolabeled with the radioisotope fluorine-18 and used as a radioligand with positron emission tomography (PET). Several research studies have used the radiolabeled setoperone in neuroimaging for the studying neuropsychiatric disorders, such as depression[1] or schizophrenia.[2]
Synthesis
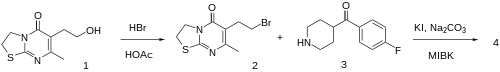
The starting material is called 6-(2-hydroxyethyl)-7-methyl-2,3-dihydro-[1,3]thiazolo[3,2-a]pyrimidin-5-one, CID:15586462 (1). Halogenation of this with hydrobromic acid in acetic acid gives CID:15586463 (2). Sn2 alkylation with 4-(4-fluorobenzoyl)piperidine [56346-57-7] (3) under Finkelstein reaction conditions affords setoperone (4).
See also
References
- ↑ Jeffrey H. Meyer, Shitij Kapur, Sylvain Houle, Jean DaSilva, Beata Owczarek, Gregory M. Brown, Alan A. Wilson and Sidney H. Kennedy (July 1, 1999). "Prefrontal Cortex 5-HT2 Receptors in Depression: An [18F]Setoperone PET Imaging Study". American Journal of Psychiatry 156 (7): 1029–1034. doi:10.1176/ajp.156.7.1029. PMID 10401447. http://ajp.psychiatryonline.org/cgi/content/full/156/7/1029.
- ↑ Ralph Lewis, Shitij Kapur, Corey Jones, Jean DaSilva, Gregory M. Brown, Alan A. Wilson, Sylvain Houle and Robert B. Zipursky (January 1, 1999). "Serotonin 5-HT2 Receptors in Schizophrenia: A PET Study Using [18F]Setoperone in Neuroleptic-Naive Patients and Normal Subjects". American Journal of Psychiatry 156 (1): 72–78. doi:10.1176/ajp.156.1.72. PMID 9892300.
- ↑ Drugs of the Future, 10, 1, 40 (1985).
- ↑ EP0070053 idem Ludo E. J. Kennis, Josephus C. Mertens, U.S. Patent 4,443,451 (1984 to Janssen Pharmaceutica N.V.).
- ↑ Maziere, B.; Crouzel, C.; Venet, M.; Stulzaft, O.; Sanz, G.; Ottaviani, M.; Sejourne, C.; Pascal, O.; Bisserbe, J.C. (1988). "Synthesis, affinity and specificity of 18F-setoperone, a potential ligand for in-vivo imaging of cortical serotonin receptors". International Journal of Radiation Applications and Instrumentation. Part B. Nuclear Medicine and Biology. 15 (4): 463–468. doi:10.1016/0883-2897(88)90018-9.
 |