Chemistry:Anacetrapib
From HandWiki
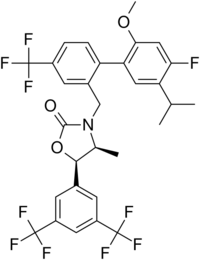
| |
| Names | |
|---|---|
| Preferred IUPAC name
(4S,5R)-5-[3,5-Bis(trifluoromethyl)phenyl]-3-{[4′-fluoro-2′-methoxy-5′-(propan-2-yl)-4-(trifluoromethyl)[1,1′-biphenyl]-2-yl]methyl}-4-methyl-1,3-oxazolidin-2-one | |
| Other names
MK-0859
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H25F10NO3 | |
| Molar mass | 637.51 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Anacetrapib is a CETP inhibitor which was being developed to treat elevated cholesterol levels in an effort to prevent cardiovascular disease.[1] In 2017 its development was abandoned by Merck.[2]
Evidence
In 2017 REVEAL trial anacetrapib was shown to decrease the risk of repeat heart attacks in high-risk patients with previous acute coronary events.[3]
See also
Other CETP inhibitors:[citation needed]
- Torcetrapib was developed by Pfizer until December 2006 but caused unacceptable increases in blood pressure and had net cardiovascular detriment.
- Dalcetrapib was developed by Hoffmann–La Roche until May 2012. It did not raise blood pressure and did raise HDL, but it showed no clinically meaningful efficacy.
- Evacetrapib was developed by Eli Lilly & Company until October 2015.
References
- ↑ "Anacetrapib, a Novel CETP Inhibitor: Pursuing a New Approach to Cardiovascular Risk Reduction". Clinical Pharmacology & Therapeutics 91 (1): 109–122. 2012. doi:10.1038/clpt.2011.271. PMID 22130116. http://www.nature.com/clpt/journal/v91/n1/full/clpt2011271a.html.
- ↑ "Merck says will not seek approval of cholesterol treatment". Reuters. 2017. https://www.reuters.com/article/us-merck-cholesterol/merck-says-will-not-seek-approval-of-cholesterol-treatment-idUSKBN1CG2W1. Retrieved 18 October 2017.
- ↑ Filippatos, TD; Kei, A; Elisaf, MS (29 September 2017). "Anacetrapib, a New CETP Inhibitor: The New Tool for the Management of Dyslipidemias?". Diseases 5 (4): 21. doi:10.3390/diseases5040021. PMID 28961179.
Further reading
- Miller, Ross A. & Aaron S. Cote, "Process for synthesizing a CETP inhibitor", WO patent 2007005572, published 2007-01-11
 |

